![1699347302923052.jpg 1.jpg]()
The development of efficient and renewable energy conversion devices is expected to alleviate the energy crisis caused by the excessive consumption of fossil fuels, and electrocatalysis can realize the mutual conversion of chemical energy and electrical energy, so it is considered to be a promising sustainable energy storage and utilization solution. At present, the correlation between catalytic activity and electrolyte cation has been widely observed in many fields of electrocatalysis, but the exact mechanism of its influence on catalytic activity is still unclear. Taking oxygen evolution reaction (OER) as an example, transition metal-based hydroxyl oxides are usually selected as efficient and economical electrocatalysts. For the electrolyte cation effect, the current research mainly focuses on the non-covalent interaction between the catalyst interface and the adsorption intermediates, however, the bulk phase structure change caused by the alkali metal cation intercalation phenomenon under the OER potential cannot be ignored. Therefore, it is particularly important to deeply understand the relationship between "dynamic structure-adsorption capacity-catalytic activity" in practical OER applications.
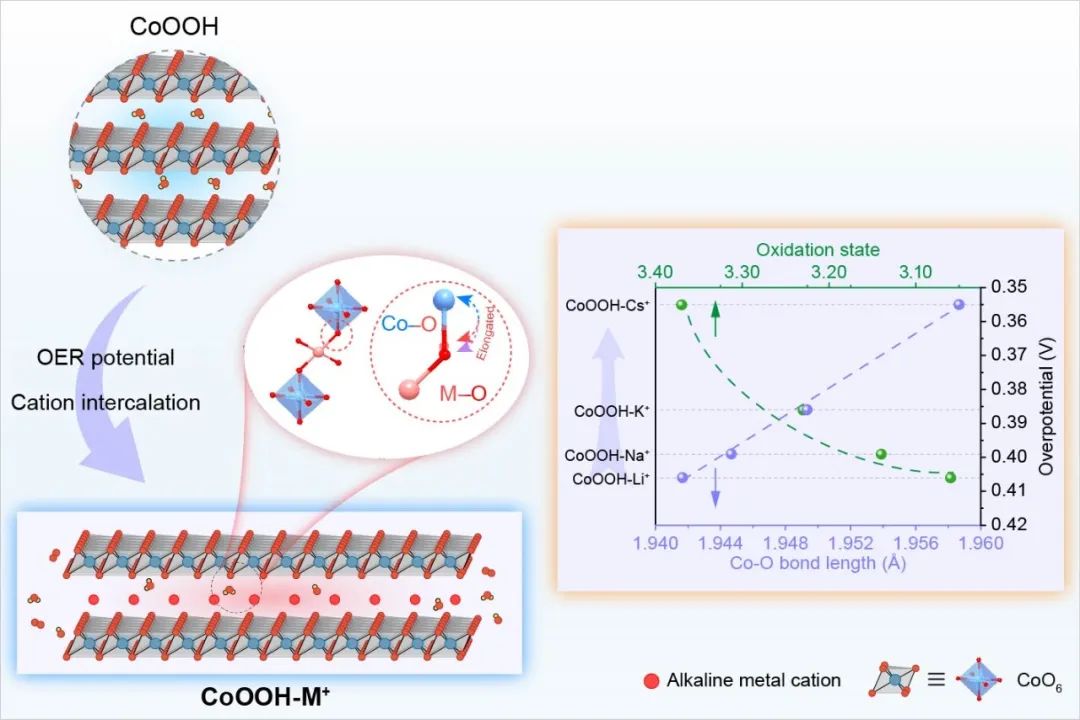
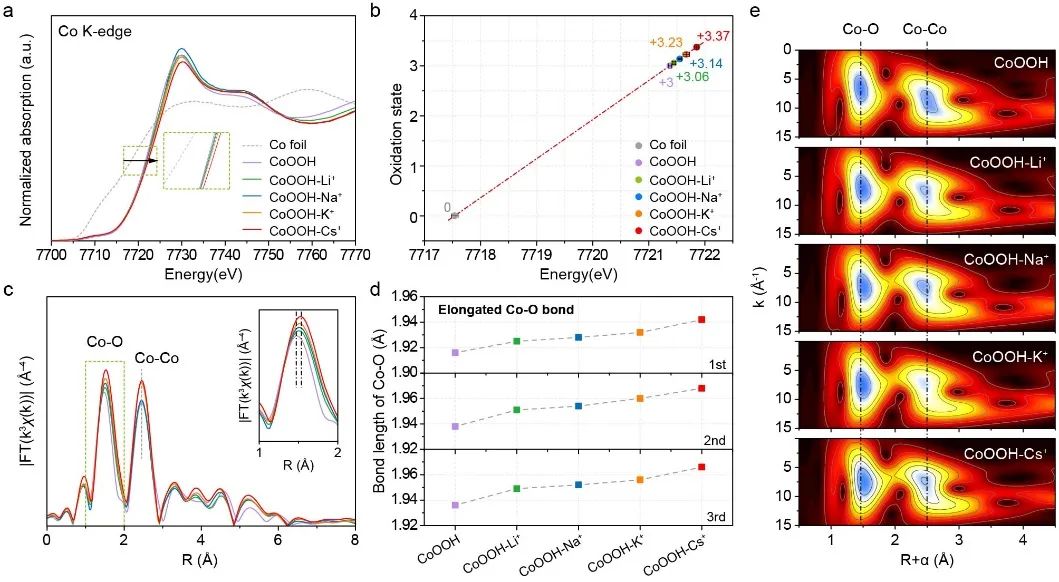
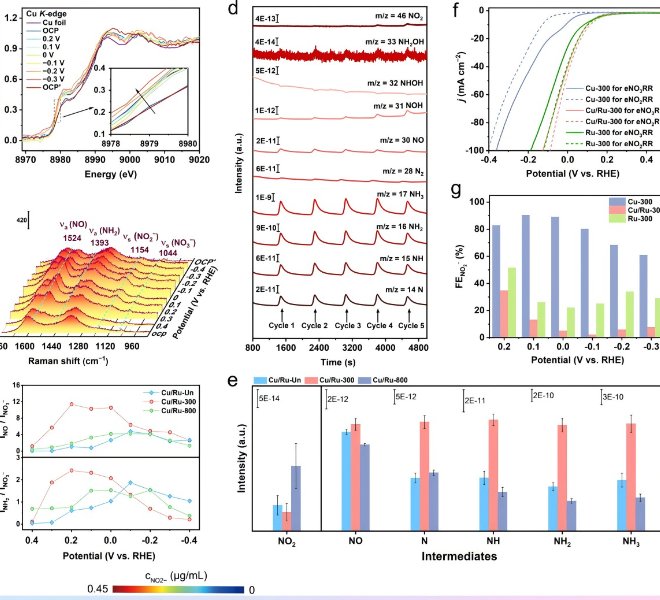
![1699347355328199.jpg 2.jpg]() Figure 1. Correlation between the microstructure of CoOOH and the catalytic activity of OER under the action of alkali metal cationsRecently, Professor Luo Wei from Wuhan University published a research article entitled "Unveiling the Electrolyte Cations Dependent Kinetics on CoOOH-Catalyzed Oxygen Evolution Reaction" in the internationally renowned journal Angewandte Chemie International Edition. In this study, the difference in the micro-environment of the catalytic site caused by alkali metal cation intercalation was taken as the starting point, and the adsorption capacity of oxygen adsorption intermediates and the difficulty of generating active sites in the OER process were correlated, and the understanding of "dynamic structure-adsorption capacity-catalytic activity" was established.Understanding and tracking the microstructural changes of CoOOH induced by electrolyte cation intercalation is critical. Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd. desktop XAFS (model: RapidXAFS 2M) helped to complete the local atomic and electronic structure characterization of CoOOH-M+. The XAFS test was completed by Wuhan students in Anhui Absorption Spectrum Instrument and Equipment Co., Ltd., and the XAFS data analysis was assisted by Anhui Absorption Spectrum staff. Fig.2 XAS analysis and structure study of CoOOH-M+The test results show that compared with other electrolyte cations with smaller ionic radius, the Cs+ ion radius in CoOOH-Cs+ is larger, and the energy of the absorption edge of Co K-edge shifts to the high-energy direction, and Co shows a higher oxidation state. Furthermore, the valence states of Co in different CoOOH-M+ were quantitatively fitted by linear extrapolation, and the average valence states of Co in each sample were +3.06 (Li+), +3.14 (Na+), +3.23 (K+) and +3.37 (Cs+), respectively, which more accurately confirmed the increase in the valence state of Co caused by cation insertion. Comparing the EXAFS spectra of the Fourier transform k3 weights corresponding to different CoOOH-M+, it was found that the length of the Co-O bond increased with the increase of the cation radius of the electrolyte. In order to describe the change of Co-O bond length more quantitatively, the first shell (~1.5Å) of the EXAFS spectrum of Co in different CoOOH-M+ was quantitatively fitted, and the fitting results further confirmed the law that the average bond length of Co-O bond increased with the increase of the cationic radius of the electrolyte. Moreover, the experimental conclusion has excellent repeatability, and the Co-O bond change law is consistent in the three repeatability experiments. With the help of the wavelet transform (WT) technique, which provides higher resolution in R space and k space and the advantage of resolving backscattered atoms, it is also found that the scattering center (1.5Å, 7.5Å-1) belonging to Co-O is slightly shifted from the initial CoOOH to CoOOH-Cs+ in the direction of high R value on the transverse axis, which is consistent with the law that the length of the Co-O bond increases with the increase of the cation radius of the electrolyte. These microstructural differences adjusted the d-band center of Co, and the d-band center of the metal Co in CoOOH-Cs+ shifted upwards most obviously, which greatly optimized the adsorption strength of oxygen intermediates in the OER process. In addition, in situ XAFS spectroscopy showed that due to the interaction between the electrolyte cation and the catalyst, it was easier to form high-valent Co species in the electrolyte with a larger cation radius, which was more conducive to inducing the formation of OER-active*O-Co(IV) species, and thus had better OER reaction kinetics.
Figure 1. Correlation between the microstructure of CoOOH and the catalytic activity of OER under the action of alkali metal cationsRecently, Professor Luo Wei from Wuhan University published a research article entitled "Unveiling the Electrolyte Cations Dependent Kinetics on CoOOH-Catalyzed Oxygen Evolution Reaction" in the internationally renowned journal Angewandte Chemie International Edition. In this study, the difference in the micro-environment of the catalytic site caused by alkali metal cation intercalation was taken as the starting point, and the adsorption capacity of oxygen adsorption intermediates and the difficulty of generating active sites in the OER process were correlated, and the understanding of "dynamic structure-adsorption capacity-catalytic activity" was established.Understanding and tracking the microstructural changes of CoOOH induced by electrolyte cation intercalation is critical. Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd. desktop XAFS (model: RapidXAFS 2M) helped to complete the local atomic and electronic structure characterization of CoOOH-M+. The XAFS test was completed by Wuhan students in Anhui Absorption Spectrum Instrument and Equipment Co., Ltd., and the XAFS data analysis was assisted by Anhui Absorption Spectrum staff. Fig.2 XAS analysis and structure study of CoOOH-M+The test results show that compared with other electrolyte cations with smaller ionic radius, the Cs+ ion radius in CoOOH-Cs+ is larger, and the energy of the absorption edge of Co K-edge shifts to the high-energy direction, and Co shows a higher oxidation state. Furthermore, the valence states of Co in different CoOOH-M+ were quantitatively fitted by linear extrapolation, and the average valence states of Co in each sample were +3.06 (Li+), +3.14 (Na+), +3.23 (K+) and +3.37 (Cs+), respectively, which more accurately confirmed the increase in the valence state of Co caused by cation insertion. Comparing the EXAFS spectra of the Fourier transform k3 weights corresponding to different CoOOH-M+, it was found that the length of the Co-O bond increased with the increase of the cation radius of the electrolyte. In order to describe the change of Co-O bond length more quantitatively, the first shell (~1.5Å) of the EXAFS spectrum of Co in different CoOOH-M+ was quantitatively fitted, and the fitting results further confirmed the law that the average bond length of Co-O bond increased with the increase of the cationic radius of the electrolyte. Moreover, the experimental conclusion has excellent repeatability, and the Co-O bond change law is consistent in the three repeatability experiments. With the help of the wavelet transform (WT) technique, which provides higher resolution in R space and k space and the advantage of resolving backscattered atoms, it is also found that the scattering center (1.5Å, 7.5Å-1) belonging to Co-O is slightly shifted from the initial CoOOH to CoOOH-Cs+ in the direction of high R value on the transverse axis, which is consistent with the law that the length of the Co-O bond increases with the increase of the cation radius of the electrolyte. These microstructural differences adjusted the d-band center of Co, and the d-band center of the metal Co in CoOOH-Cs+ shifted upwards most obviously, which greatly optimized the adsorption strength of oxygen intermediates in the OER process. In addition, in situ XAFS spectroscopy showed that due to the interaction between the electrolyte cation and the catalyst, it was easier to form high-valent Co species in the electrolyte with a larger cation radius, which was more conducive to inducing the formation of OER-active*O-Co(IV) species, and thus had better OER reaction kinetics. ![Image]()
This work not only provides insights into the OER dynamics of electrolyte cation dependence, but also provides clues to understand the mechanism of targeted electrocatalysis by other electrolyte cations.
Anhui Absorption Spectrum Instrument Equipment Co., Ltd. is led by academicians, based on the background of synchrotron radiation in the field of absorption/emission spectrum of more than 10 years of technical research accumulation, the development of standardized desktop X-ray absorption/emission spectrum equipment. Focusing on the development of X-ray absorption/emission spectrum technology and spectroscopy instruments, providing professional absorption/emission spectrum technology solutions for scientific researchers, adhering to the technical pursuit of "bringing XAFS into the laboratory", delving into absorption/emission spectrum technology, carrying forward the spirit of craftsmanship and modern scientific innovation, and persistently promoting the research and development of X-ray technology and equipment. As a professional XAFS person, we have been making professional XAFS equipment and are committed to helping you XAFS freedom.