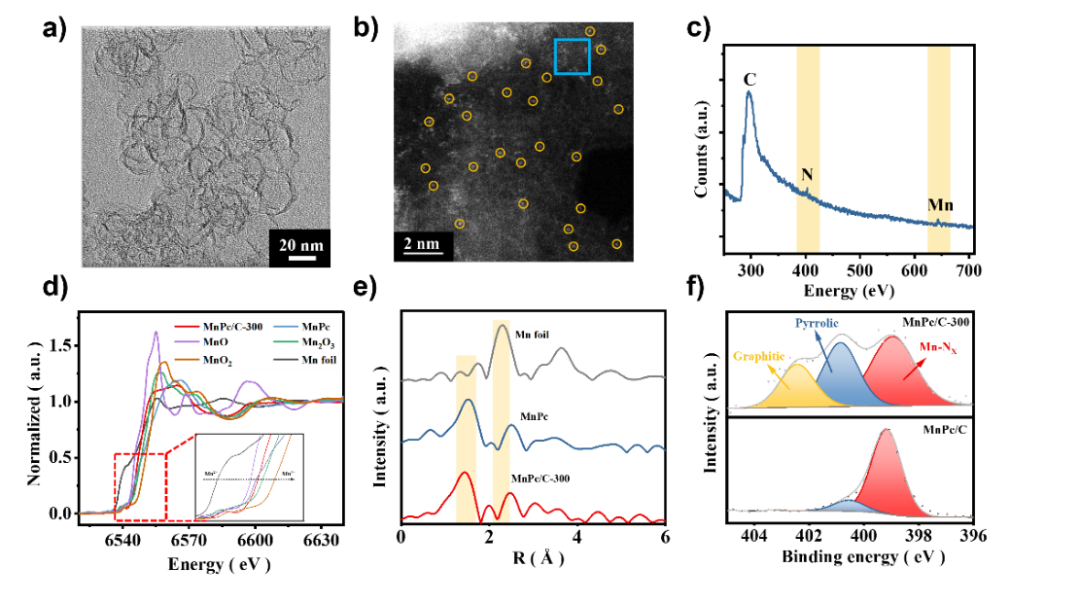
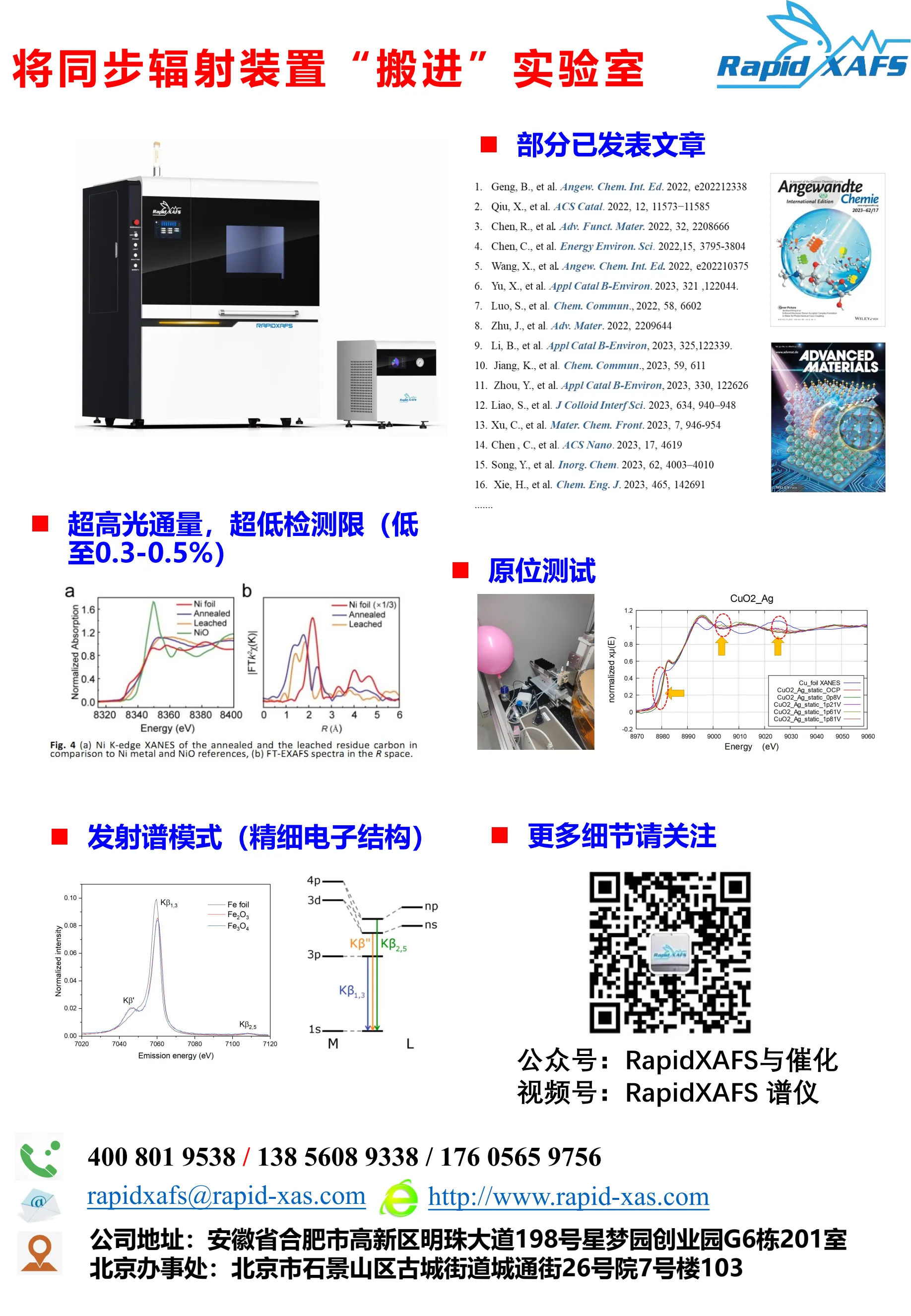
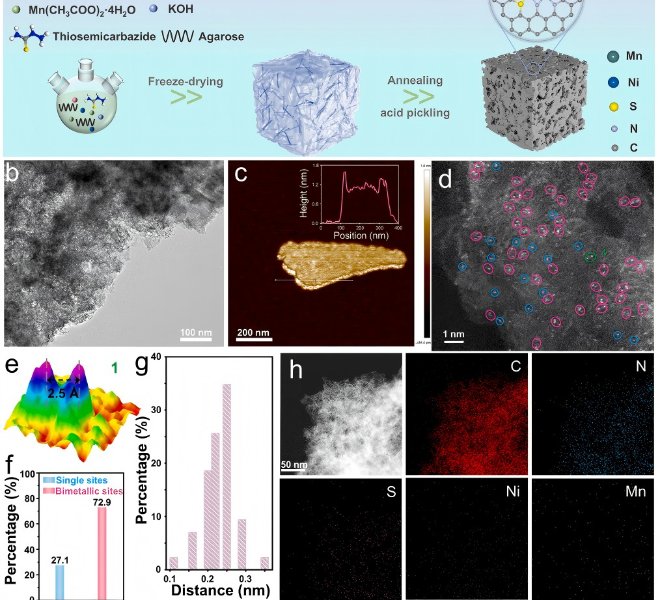
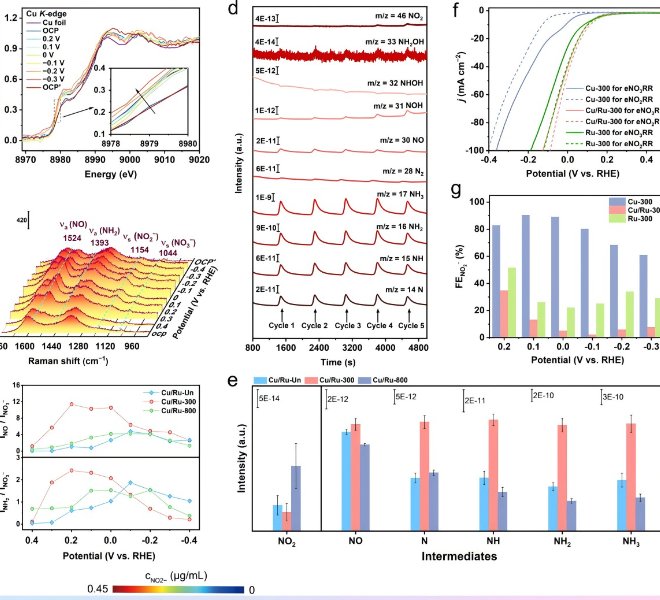
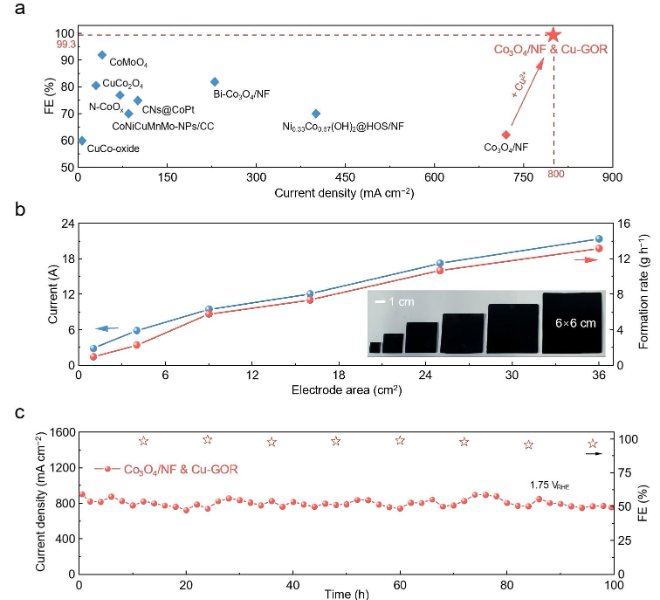
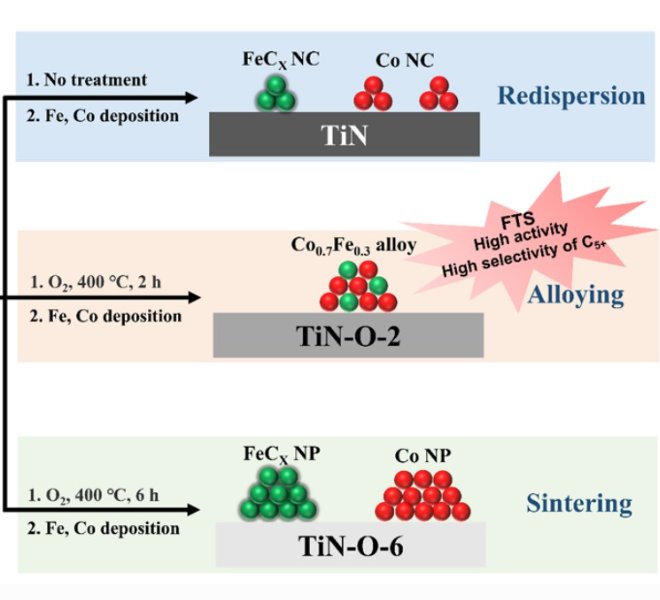
The benchtop XAFS (model: RapidXAFS 2M) facilitated the structural characterization of the M-N-C catalyst, and the scientific research results were published in the form of papers in the international journal Appl. Catal. Environ.: "Unraveling the role of hydrogen peroxide in the pH-dependent ORR performance of Mn-N-C catalysts".
![1700192681843427.jpg 1.jpg]()
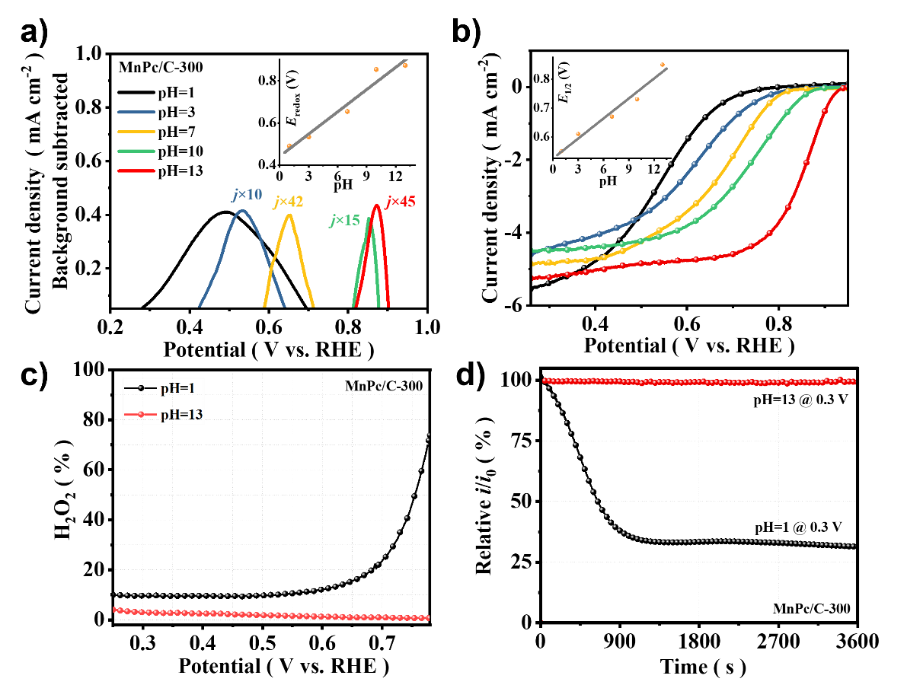
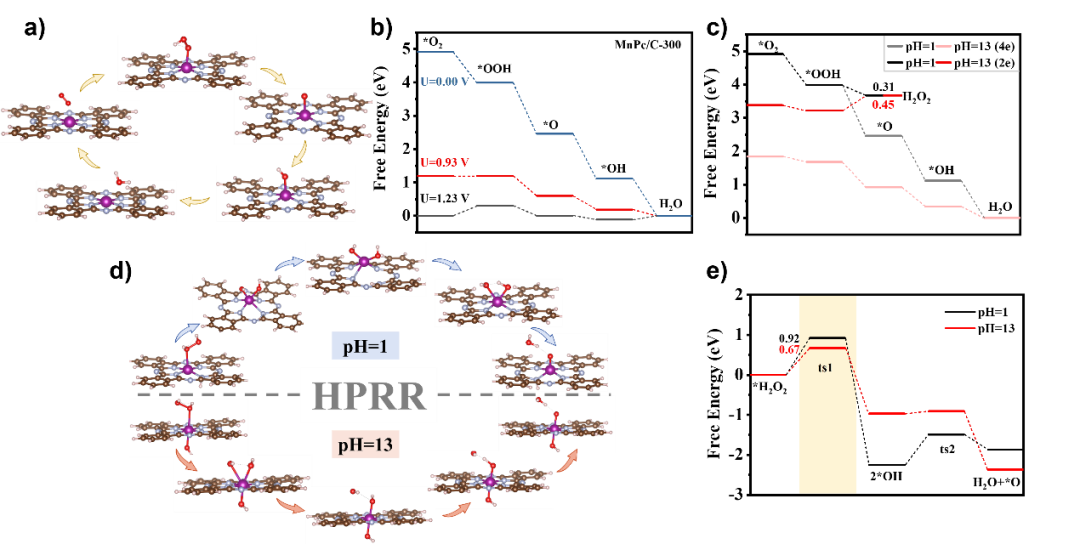
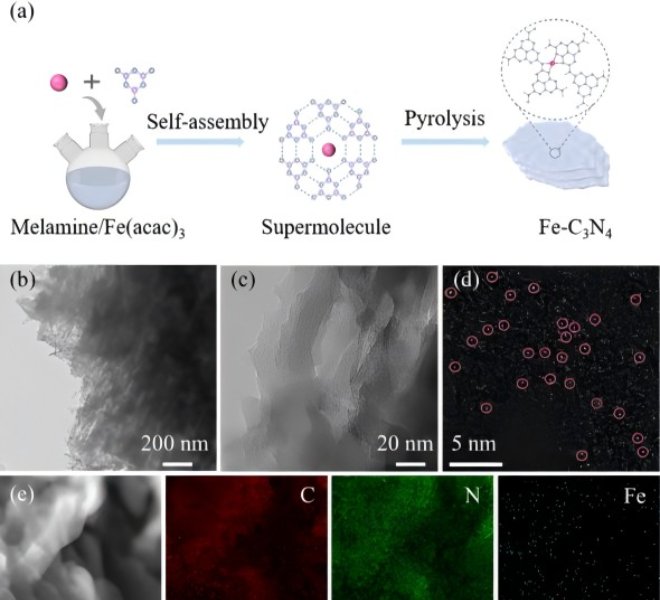
The Fe-N-C transition metal catalyst is considered to be the most promising platinum group metal (PGM)-free ORR catalyst under proton exchange membrane fuel cell (PEMFC) operating conditions, exhibiting a performance loss of more than 50% in the first 100 hours due to its poor electrochemical stability, well below the minimum requirements for portable power applications, creating an excellent opportunity to find novel pyrolysis catalysts with low Fenton reactivity in addition to the Fe-N-C catalyst. Mn-N-C, as a catalyst with lower Fenton reactivity, has made significant progress in synthesis technology and performance improvement. So far, the Mn-N-C catalyst has shown high activity in alkaline media, but poor performance in acidic media. Understanding its interesting but confusing strong pH-dependent activity facilitates the rational design of high-performance Mn-N-C catalysts.近日,郑州大学卢帮安副教授与张佳楠教授在国际期刊Applied Catalysis B: Environmental上发表了题为“Unravelling the role of hydrogen peroxide in pH-dependent ORR performance of Mn-N-C catalysts”的文章。Since the manganese phthalocyanine molecule (MnPc) has a similar and well-definedMnN4 active site structure to the actual pyrolysis Mn-N-C catalyst, it makes it an ideal model catalyst for deriving the pH effect of such catalysts. In this study, Mn-N-C catalysts were synthesized by loading MnPc molecules onto porous carbon black and then heat treatment. The coordination structure of the active center was illustrated by electron microscopy and XAFS spectroscopy. Firstly, the high-resolution HAADF-STEM and EELS spectra were used to confirm the definite existence of Mn and the uniform dispersion at the atomic level. However, its specific structure still needs to be confirmed, and further XAFS technology is used to analyze its local structure characteristics in detail, and all the XAFS data in this experiment are measured by the desktop X-ray absorption spectrum of Anhui Absorption Spectrum Instrument Equipment Co., Ltd., and the model is RapidXAFS 2M. From the XANES of Mn k-edge, it is known that the absorption side energy of the MnPc/C-300 catalyst is between the reference samples MnO andMn2O3, indicating that the chemical state of Mn in MnPc/C-300 is between +2 valence and +3 valence. By performing the Fourier transform on the XAFS of MnPc/C-300, it was found that its overall characteristics were similar to that of the standard MnPc, and the coordination peak attributed to the Mn-N coordination was exhibited in the 1.44Å attachment, which was an approximate match to the Mn-N in the standard MnPc. However, no signal was observed at the position of the Mn-Mn coordination position of the characteristic metal Mn-Mn in the ~2.3Å annex, indicating that there was no Mn in the particle form of the MnPc/C-300 catalyst, which was consistent with the results of high-resolution electron microscopy. In addition, the coordination number of Mn-N in the MnPc/C-300 catalyst is 3.3 and the bond length is 1.94, which further indicates that most of the Mn in the catalyst still maintains the local characteristic structure of Mn-N4. XAFS technology was used to unequivocally confirm that Mn is atomic-level dispersed, embedded in the support, and coordinated with four N atoms in the vicinity in the MnPc/C-300 catalyst. ![1700192757851618.jpg 2.jpg]() Fig. 1. The morphology and structure of catalysts. (a) TEM image of MnPc/C-300; (b) AC-HAADF-STEM of Mn single atom of MnPc/C-300 catalyst and (c) EELS of the recorded areas shown in Figure b; (d) The experimental K-edge XANES spectra of MnPc/C-300 catalyst and reference sample (Mn foil, MnO, Mn2O3, MnO2, MnPc); (e) Fourier transform of Mn-K edge EXAFS data for MnPc/C-300, MnPc and Mn Foil catalysts; (f) High-resolution N 1s XPS spectra of MnPc/C and MnPc/C-300 catalysts.This article provides a new understanding of the role of hydrogen peroxide in the pH-dependent activity and stability of Mn-N-C catalysts. In alkaline and acidic media, · The affinity of OH for the Mn active center varies greatly, and the significant effect of pH on ORR activity is the result of theH2O2reduction reaction (HPRR) kinetics in alkaline media being significantly faster than in acidic media. The rate of ORR HPRR is controlled by pH-dependent oxidation of the central surface state of Mn. The kinetics of HPRR may stem from the intrinsic affinity of the surface for oxygenated species.Fig. 2. The pH-dependent ORR performance. (a) SWV of MnPc/C-300 catalyst in N2-saturated electrolyte with different pH. Inset: The fit curve of pH and Eredox. (b) Polarization curve of MnPc/C-300 catalyst in O2-saturated electrolyte with different. Inset: The fit curve of pH and E1/2.According to the DFT calculations, the dissociation of the HO-OH bond is facilitated in an alkaline environment, resulting in a reduction in the energy required to formH2O. For H2O2-induced inactivation, considerable pH dependence has been observed in terms of its durability. Corresponding to the loss of the active site, acidicH2O2treatment resulted in a significant decrease in activity, while alkaline treatment resulted in little to no inactivation.Fig. 3. (a) The proposed ORR mechanism on MnN4 sites; (b) Gibbs free-energy diagrams on MnN4; (c) Gibbs free-energy diagrams of ORR on MnN4 under 0 V at different pH; (d) Proposed HPRR mechanism on MnN4 sites at different pH; (e) Gibbs free-energy diagrams of HPRR on MnN4 at pH=1 and pH=13.
Fig. 1. The morphology and structure of catalysts. (a) TEM image of MnPc/C-300; (b) AC-HAADF-STEM of Mn single atom of MnPc/C-300 catalyst and (c) EELS of the recorded areas shown in Figure b; (d) The experimental K-edge XANES spectra of MnPc/C-300 catalyst and reference sample (Mn foil, MnO, Mn2O3, MnO2, MnPc); (e) Fourier transform of Mn-K edge EXAFS data for MnPc/C-300, MnPc and Mn Foil catalysts; (f) High-resolution N 1s XPS spectra of MnPc/C and MnPc/C-300 catalysts.This article provides a new understanding of the role of hydrogen peroxide in the pH-dependent activity and stability of Mn-N-C catalysts. In alkaline and acidic media, · The affinity of OH for the Mn active center varies greatly, and the significant effect of pH on ORR activity is the result of theH2O2reduction reaction (HPRR) kinetics in alkaline media being significantly faster than in acidic media. The rate of ORR HPRR is controlled by pH-dependent oxidation of the central surface state of Mn. The kinetics of HPRR may stem from the intrinsic affinity of the surface for oxygenated species.Fig. 2. The pH-dependent ORR performance. (a) SWV of MnPc/C-300 catalyst in N2-saturated electrolyte with different pH. Inset: The fit curve of pH and Eredox. (b) Polarization curve of MnPc/C-300 catalyst in O2-saturated electrolyte with different. Inset: The fit curve of pH and E1/2.According to the DFT calculations, the dissociation of the HO-OH bond is facilitated in an alkaline environment, resulting in a reduction in the energy required to formH2O. For H2O2-induced inactivation, considerable pH dependence has been observed in terms of its durability. Corresponding to the loss of the active site, acidicH2O2treatment resulted in a significant decrease in activity, while alkaline treatment resulted in little to no inactivation.Fig. 3. (a) The proposed ORR mechanism on MnN4 sites; (b) Gibbs free-energy diagrams on MnN4; (c) Gibbs free-energy diagrams of ORR on MnN4 under 0 V at different pH; (d) Proposed HPRR mechanism on MnN4 sites at different pH; (e) Gibbs free-energy diagrams of HPRR on MnN4 at pH=1 and pH=13.
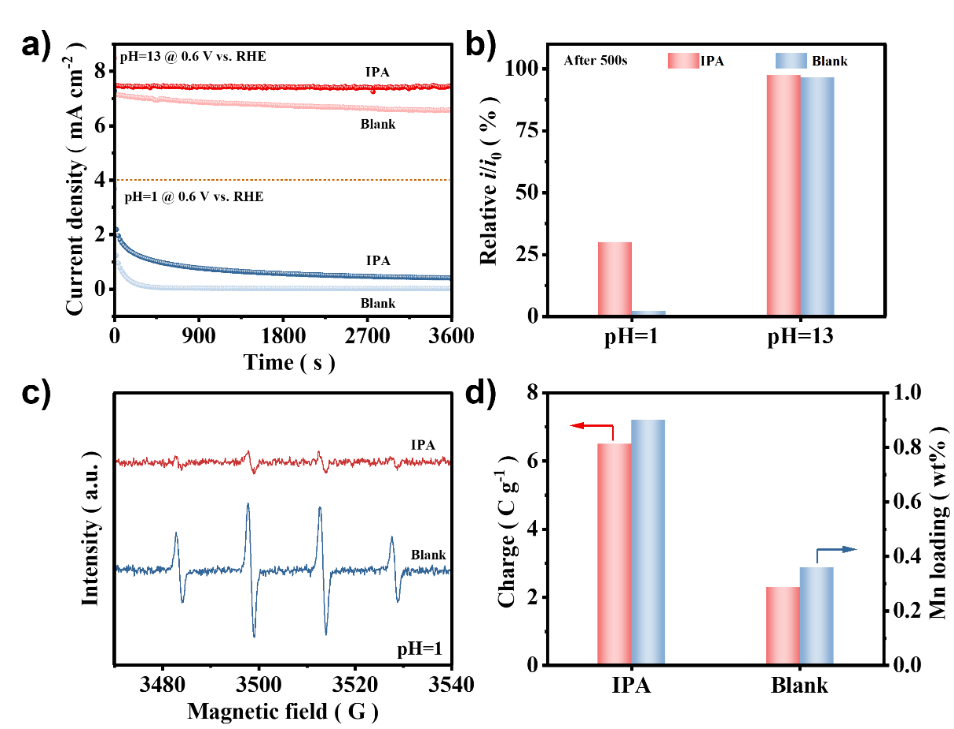
In addition, the study used isopropanol (IPA) as a rapid scavenger of reactive oxygen species (ROS) formed near the active site, resulting in a significant increase in the stability of the catalyst in acidic and alkaline media.Fig. 7. The effect of hydroxy radical scavenger. (a) Chronoamperometry test of MnPc/C-300 catalyst operating at 0.6 V with pH=13 and pH=1 electrolyte with IPA and without IPA; (b) the relative current retention ratio of MnPc/C-300 catalyst at pH=13 and pH=1 electrolyte with IPA and without IPA after chronoamperometry test 500 s; (c) the EPR spectra of MnPc/C-300 catalyst with IPA and without IPA used 0.1 M HClO4 as solvent; (d) the amount of desorption charge and Mn loading of MnPc/C-300 catalyst at electrolyte pH=1 after chronoamperometry test with IPA and without IPA.Anhui Absorption Spectrum Instrument Equipment Co., Ltd. is led by experts, based on the background of synchrotron radiation in the field of absorption/emission spectrum of more than 10 years of technical research accumulation, the development of standardized desktop X-ray absorption/emission spectrum equipment. Focusing on the development of X-ray absorption/emission spectrum technology and spectroscopy instruments, providing professional absorption/emission spectrum technology solutions for scientific researchers, adhering to the technical pursuit of "bringing XAFS into the laboratory", delving into absorption/emission spectrum technology, carrying forward the spirit of craftsmanship and modern scientific innovation, and persistently promoting the research and development of X-ray technology and equipment. As a professional XAFS person, we have been making professional XAFS equipment and are committed to helping you XAFS freedom.