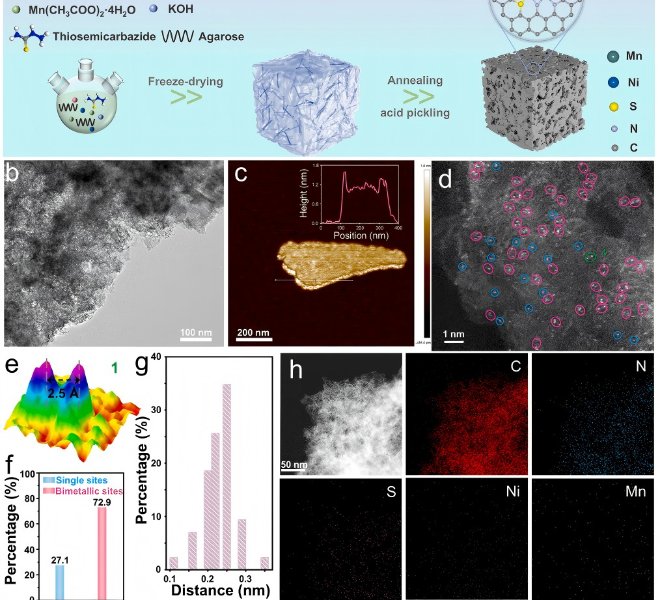
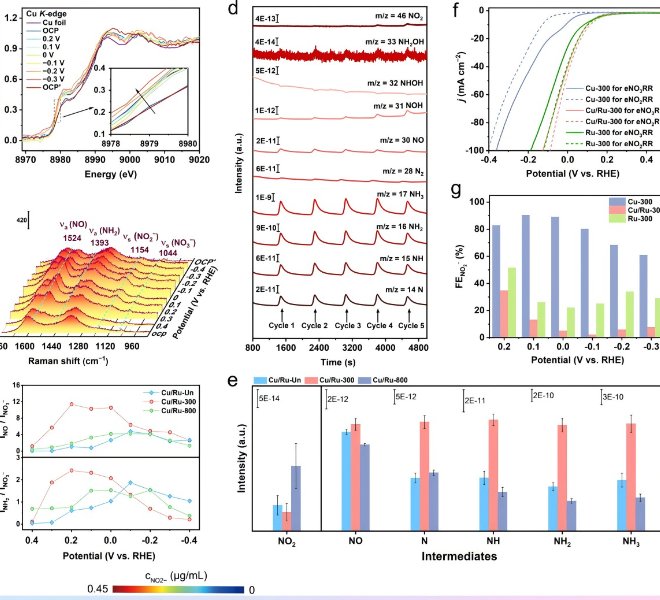
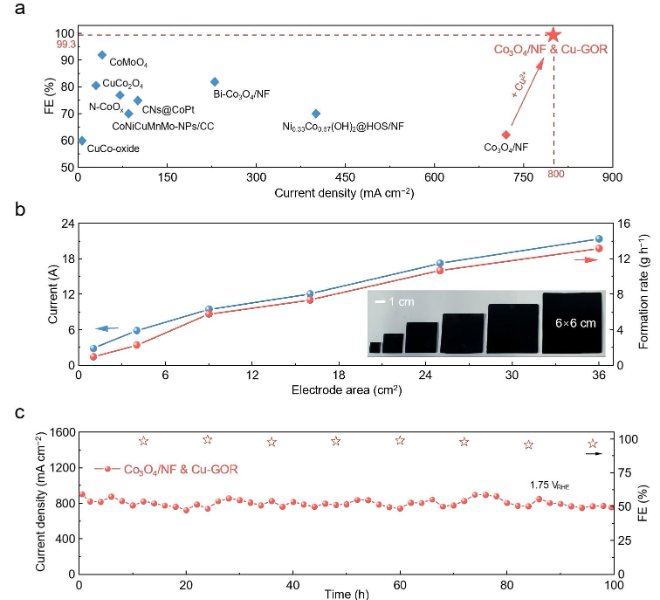
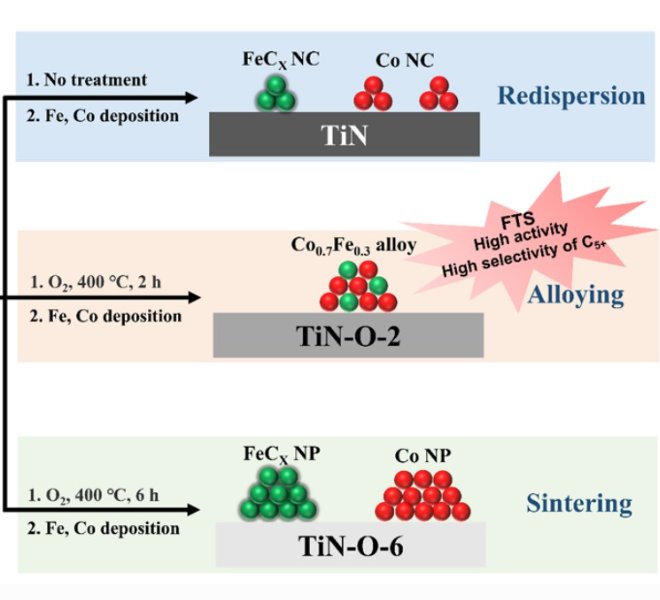
The benchtop XAFS (model: RapidXAFS 2M) independently developed by Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd. assisted in the structural characterization of Se-doped NiTe catalysts, and the scientific research results were published in the form of a paper in the international journal Journal of Materials Chemistry A: "One-step hydrothermal synthesis of Se-doped NiTe electrocatalysts for efficient hydrogen production from anode iodine oxidation-assisted brine".
![1700534569815495.png 1.png]()
The direct electrolysis of seawater from renewable energy to produce green hydrogen has significant economic advantages, and the high concentration of chloride ions in seawater is prone to chlorine oxidation competition reaction at high voltage, which not only affects the efficiency of the anode Faraday, but also produces corrosive and environmentally unfriendly substances, which in turn affects the life of each component of the electrolyzer. Theoretically, there is only a potential difference of 0.48 V between the oxygen evolution reaction (OER) and the chlorine evolution reaction (CER) of alkaline electrolyzed water, and the triggering of OER often requires a large overpotential, so it is difficult to avoid the problem of chlorine precipitation. In order to minimize the disadvantages caused by chlorine oxidation, the anode can be significantly reduced by using non-interfering small molecule oxidation (SMOR) instead of OER.
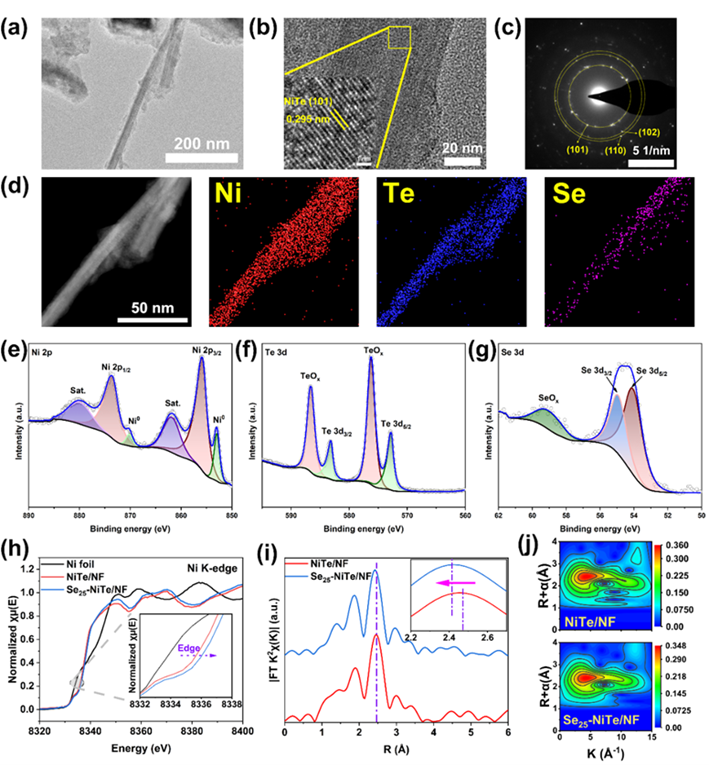
Recently, researcher Liu Lifeng of Songshan Lake Materials Laboratory and researcher Xiong Dehua of Wuhan University of Technology published a paper entitled ""One-step hydrothermal synthesis of Se-doped NiTe electrocatalysts for efficient hydrogen production from saline water assisted by the anodic iodide oxidation" article. In this paper, a selenium-doped nickel telluride nanoarray electrode (Se-NiTe/NF) was developed on nickel foam current collectors by one-step hydrothermal method, and the optimized Se-NiTe/NF has abundant catalytic active sites, which is helpful to improve the OER performance in the alkaline simulated seawater electrolyte (1.0 M KOH+0.5 M NaCl). In addition, an iodide ion oxidation (IOR) with a low theoretical oxidation potential was noted and applied to the electrolysis simulation of renewable energy in seawater, which only required an anode potential of 1.376 V vs RHE to reach 100 mA cm-2and continuously catalyze IOR for 500 hours at this current density. The coupling cathodic hydrogen evolution reaction (HER) effectively reduces the energy consumption of the electrolyzer, and at the same time obtains high value-added iodine derivatives at the anode. This work shows that combining HER with IOR represents a promising method for hydrogen production, while upgrading iodide ions from low-cost, abundant and renewable seawater to a value-added commodity, also helps to address the long-term challenges of chlorine precipitation, enabling seawater electrolysis to take place at high current densities over the long term. The high-resolution TEM and SAED were used to confirm the uniform distribution of various elements, and the HAADF-STEM images further verified the lattice spacing that still belongs to NiTe after Se doping. The high-resolution XPS spectra of Ni 2p and Te 3d show the characteristic peaks of Ni0 and Te0, respectively, indicating the formation of Ni-Te bonds. The local electronic and coordination structures of the catalyst were further clarified by XANES and EXAFS technologies. All the XAFS data in this experiment were measured by the desktop X-ray absorption spectroscopy of Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd., and the model was RapidXAFS 2M. XANES spectra according to Ni K-edge show that the introduction of Se changes the charge distribution of the metal Ni and thus the oxidation state of the Ni site, Se25-The energy of the absorption edge in -NiTe/NF shifts slightly to higher energies, and the intensity of the white line peak increases accordingly, indicating that Ni has more unoccupied states and higher valence states, which indicates that there is an electron transfer from Ni to Se/Te, which is further supported by the XPS characterization of the Ni0 peak to the high energy offset. The EXAFS spectra of the Fourier transform showed strong peaks at R≈2.5Å, which could be attributed to the bonding between Ni-Te. Further analysis showed that after Se doping, which has stronger electronegativity, attracted surrounding atoms and induced Ni-Te bond reconstitution, resulting in a shortening of the Ni-Te bond length. At the same time, the wavelet transform method is used to further discuss the XAFS data in R space and k space, and the scattering centers are similar before and after the introduction of Se, and there is only a shift in the R direction, which is consistent with the above conclusions. Summarizing the above analyses, the introduction of Se into NiTe does change the electronic and ligand structure of Ni, which is conducive to the occurrence of OER/IOR electrocatalytic reactions. ![1700534604622727.jpg 2.jpg]()
Fig. 2 (a) TEM image, (b) HRTEM image. (c) SAED pattern. (d) HAADF-STEM image and EDX elemental maps of the Se25-NiTe electrocatalyst. High-resolution XPS spectra of (e) Ni 2p, (f) Te 3d, and (g) Se 3d core-levels of the Se25-NiTe/NF electrode. (h) Ni K-edge XANES spectra. (i) Fourier transforms of the k2 weighted EXAFS oscillations in R-space. (j) Wavelet transform contour maps of the Ni K-edge EXAFS, derived from for Se25-NiTe and NiTe powders.
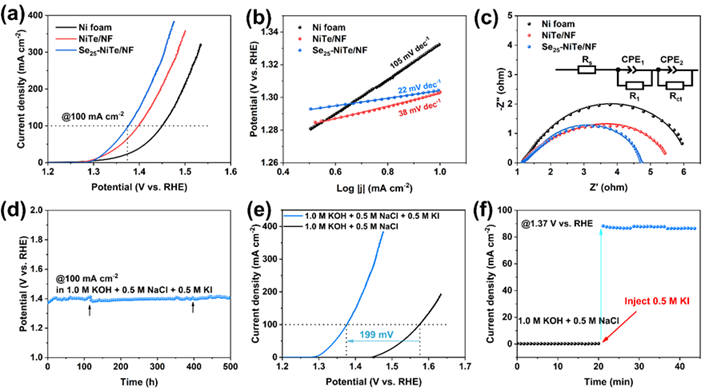
The authors added a concentration of KI to a simulated seawater electrolyte (1.0 M KOH + 0.5 M NaCl) for catalytic performance evaluation. It can be seen that the Se-NiTe/NF electrode is able to provide 100 mA cm−2 at a potential of 1.376 V vs RHE, showing excellent IOR activity. Notably, in the presence of KI, the electrode exhibits excellent long-term stability in simulated seawater electrolysis, capable of continuously catalyzing IOR for up to 500 hours at 100 mA cm-2. In alkaline solutions, the end product of iodine ion oxidation is iodate ions, and the whole IOR is an electrochemical-chemical (E-C) process in which I− is first oxidized to I2, which is unstable in alkaline and spontaneously disproportionated to I−and IO3−. The color change of the electrolyte before and after the reaction and the Raman spectroscopy data clearly showed that IO3− was the final product of IOR in the alkaline brine solution.![1700534629939890.png 3.png]()
Fig. 5 Electrochemical IOR test results of Ni foam, NiTe/NF and Se25-NiTe/NF electrodes in 1.0 M KOH + 0.5 M NaCl + 0.5 M KI electrolyte. (a) Cyclic voltammetry curves. (b) Tafel plots. (c) Nyquist plots. (d) Chronopotentiometry plot (The arrows indicate the replenishments of the electrolyte) of the Se25-NiTe/NF electrode. (e) Polarization curves in the absence and presence of 0.5 M KI and (f) Chronoamperometry plot for the Se25-NiTe/NF electrode.
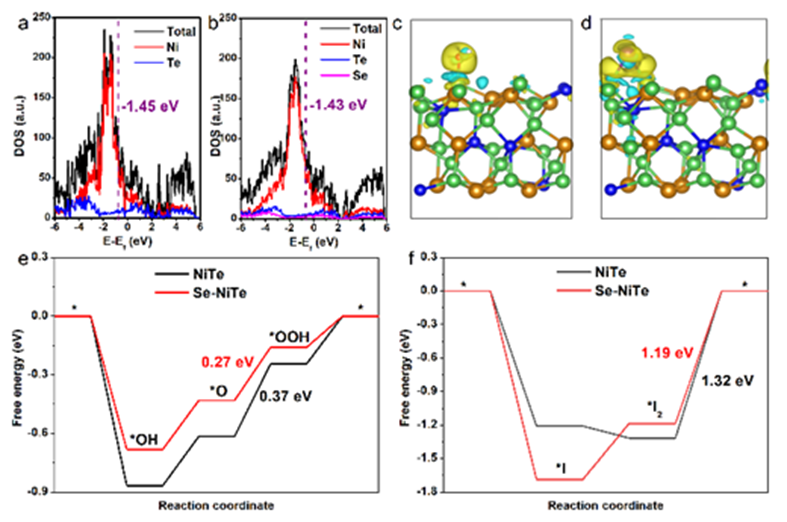
Finally, in order to explore the reason for the increase in activity caused by doping, according to the DFT calculation, Ni is the catalytic activity center, and compared with the original NiTe, the d-band center of Se-NiTe is shifted upward, which will provide more efficient electron transfer for OER and IOR. In addition, the results of charge density distribution during the adsorption of reaction intermediates showed that the electron density around the intermediates adsorbed on Se-NiTe was higher, indicating that the electron transfer from the Ni site to the intermediates was more significant when Se was doped, which was conducive to the adsorption and activation of the intermediates, thereby improving the OER/IOR reaction kinetics. The Gibbs free energy changes of the OER reaction pathway show that the potential determination step (PDS) is *O → *OOH, and the PDS energy barrier decreases from a relatively high energy barrier of 0.37 eV to 0.27 eV after the introduction of Se, indicating that Se doping can help to improve the intrinsic OER activity. Similarly, according to the Gibbs free energy change of the IOR, the desorption of I2 becomes PDS, and the energy barrier of Se-NiTe to PDS is significantly smaller than that of the original NiTe, which confirms that Se doping also has a promoting effect on IOR.![1700534651564737.jpg 4.jpg]() Fig. 7 Calculated DOS profiles of (a) NiTe and (b) Se-NiTe. The structural model and the corresponding charge density difference on Se-NiTe toward the (c) OH* and (d) I* adsorption. The green, brown, blue, red, white and purple spheres represent Ni, Te, Se, O, H and I, respectively. The yellow or cyan regions indicate the accumulation or depletion of the charges, respectively. Gibbs free-energy diagrams of the (e) OER and (f) IOR.
Fig. 7 Calculated DOS profiles of (a) NiTe and (b) Se-NiTe. The structural model and the corresponding charge density difference on Se-NiTe toward the (c) OH* and (d) I* adsorption. The green, brown, blue, red, white and purple spheres represent Ni, Te, Se, O, H and I, respectively. The yellow or cyan regions indicate the accumulation or depletion of the charges, respectively. Gibbs free-energy diagrams of the (e) OER and (f) IOR.Anhui Absorption Spectrum Instrument Equipment Co., Ltd. is led by experts, based on the background of synchrotron radiation in the field of absorption/emission spectrum of more than 10 years of technical research accumulation, the development of standardized desktop X-ray absorption/emission spectrum equipment. Focusing on the development of X-ray absorption/emission spectrum technology and spectroscopy instruments, providing professional absorption/emission spectrum technology solutions for scientific researchers, adhering to the technical pursuit of "bringing XAFS into the laboratory", delving into absorption/emission spectrum technology, carrying forward the spirit of craftsmanship and modern scientific innovation, and persistently promoting the research and development of X-ray technology and equipment. As a professional XAFS person, we have been making professional XAFS equipment and are committed to helping you XAFS freedom.