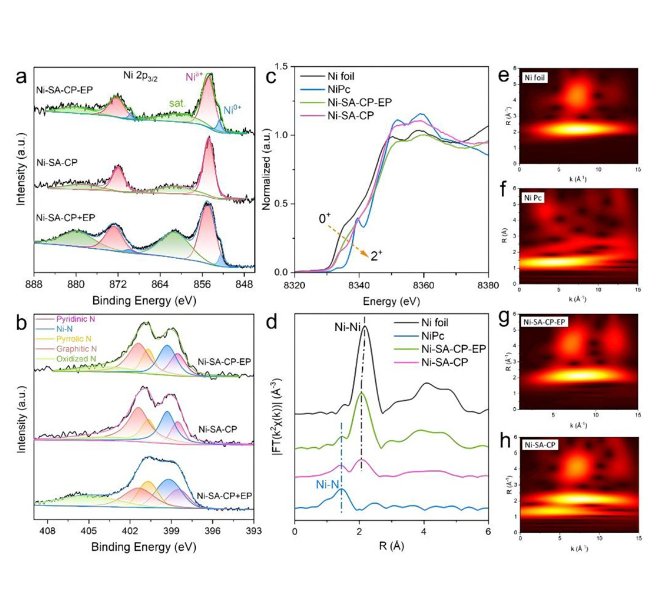
In-situ spectroscopy characterized the sub-nanometer CoOx cluster catalysts (CoOx@MFI) encapsulated by MFI zeolite, which exhibited good catalytic performance in PDH reactions
Propylene is one of the most important organic chemical raw materials. Driven by the extensive exploitation of shale gas resources, propane dehydrogenation (PDH) is an effective alternative to naphtha steam cracking processes to produce propylene. But currently industrial catalysts PtSn/Al2O3 and CrOx/Al2o3 face significant challenges due to excessive costs and harmful effects on the environment, respectively.
In view of this, Yongan Yang from Tianjin University, Xi Wang from Beijing Jiaotong University, and Xuejing Zhang from Jiangsu University of Science and Technology reported a simple in-situ synthesis method for the preparation of sub-nano CoOx cluster catalysts (CoOx@MFI) encapsulated by MFI zeolite, which exhibited good catalytic performance in PDH reactions.
Point 1: The optimized catalyst has excellent catalytic performance for propane dehydrogenation, with a propane conversion rate of 59% and propylene selectivity of 93% (600 °C).
Point 2: In-situ spectroscopy and theoretical calculations show that the stable Co−O group of zeolite plays a key role in activating C−H bonds, and verifies the reaction path of O atoms as Lewis bases to pull the dropped H atoms to generate [CoOH]+ intermediates.
Figure 1. Synthesis and characterization of CoOx@MFI catalysts. (a) Synthesis protocols. (b) Infrared spectra of catalysts with different metal loads. (c) 29Si MAS NMR of catalysts with different metal loadings. (d) H2-TPR for CoOx@MFI and Co3O4/MFI.
A series of CoOx@MFI catalysts encapsulated by MFI zeolites were prepared by in-situ synthesis, and combined with XRD and FT-IR spectroscopy, it was found that the introduction of CoOx species with different loads did not cause structural changes in MFI zeolite, and they were uniformly dispersed in the zeolite, forming a large number of Co-O-Si structural units. Compared with theCo3O4/MFI catalyst, this in-situ synthesis catalyst requires a higher reduction temperature, and it is difficult for Co species to be reduced.
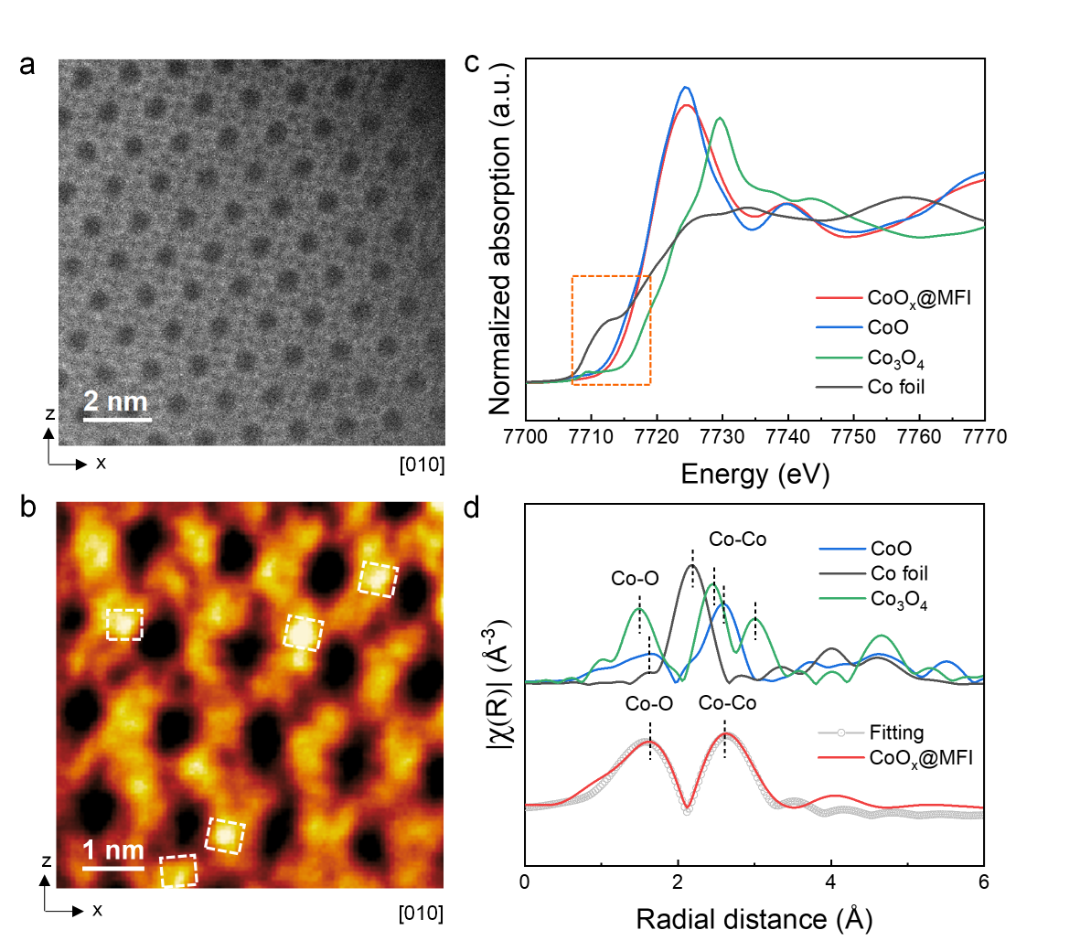
Figure 2. Fine structure of Co in CoOx@MFI catalysts. (a-b) Spherical aberration corrected HAADF-STEM images and corresponding iDPC-STEM images. (c) Co k-edge XANES map. (d) K3-weighted EXAFS profile of the CoOx@MFI catalyst.
Spherical aberration electron microscopy (PEM) confirmed that these sub-nanometer CoOx clusters (0.4-0.6 nm) preferred to settle in the sinusoidal pores of the MFI zeolite rather than in the through pores. Furthermore, in order to more accurately analyze the coordination structure of Co species in molecular sieves, synchrotron radiation XAS measurements were performed on the CoOx@MFI. All XAFS data in this experiment were measured by the desktop X-ray absorption spectrometer of Anhui Absorption Spectroscopy Instrument and Equipment Co., Ltd., and the model was RapidXAFS 2M. Compared with the normalized Co k-edge XANES spectrum, it is found that the oscillation characteristics are similar to those of Co3O4 and CoO standard spectra, and the absorption edge energy is between Co3O4 and CoO, indicating that the oxidation state of Co in the CoOx@MFI is between 2 and 3 (Co3−δ, 0 < δ < 1), which is consistent with the corresponding XPS analysis results. Compared with the EXAFS spectra of Fourier transform, the Co-O coordination peaks belonging to the first shell and the Co-Co coordination peaks belonging to the second shell were found, with bond lengths of 2.05 and 3.04 Å, respectively. This means that Co is located within the MFI channel as a cluster of CoOs rather than isolated Co species, which is consistent with the conclusions of previous aberration-corrected HAADF-STEM and wavelet transform plots. The coordination numbers of Co-O, Co-Co and Co-O-Si reached 3.9, 2.9 and 6.1, respectively, indicating that the size of the CoOx cluster was slightly larger, and the Co-O-Si of the third shell was enhanced by SMSI. Based on these detailed analyses, it was confirmed that this strategy of in situ synthesis can successfully embed CoOx clusters (Co-O groups) into the MFI zeolite pores.
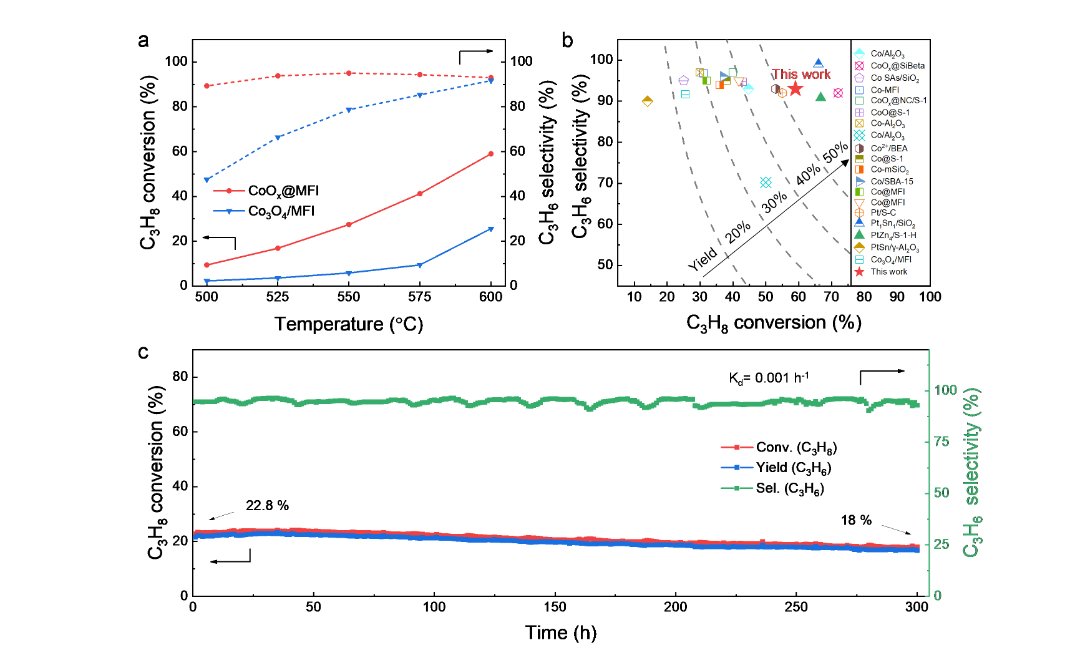
Figure 3. Catalytic performance of PDH reactions. (a) C3H8 of the temperature for CoOx@MFI and Co3O4/MFI catalysts Conversion rate and selectivity impact. WHSV = 6000 mL g−1 h−1,T = 600 ℃。 (b) Comparison of catalytic performance. (c) Stability of CoOx @MFI catalysts. T = 550 ℃。
By optimizing the CoOx@MFI Co loading, the volcanic trend was achieved, and at 600°C,the C3H8 conversion rate of 5 wt % CoOx@MFI was the highest (59.0%), which exceeded the Co3O4/ of the same Co loading. MFI sample (approx. 28%). C3H6 achieved 90% selectivity on both catalysts. But it is clear that PDH on theCO3O4/MFI catalyst requires a higher temperature. CoOx@MFI catalyst has irreducible Co-O groups, and its performance is better than that of previously reported Co-based, Pt-based catalysts and traditional PtSn/Al2O3 catalysts. CoOx@MFI catalyst also has very excellent stability, even in the case of 300h operation, it can still achieve 18% C3H8conversion rate and 93.0% propylene selectivity, and the inactivation rate kd~0.001h-1。
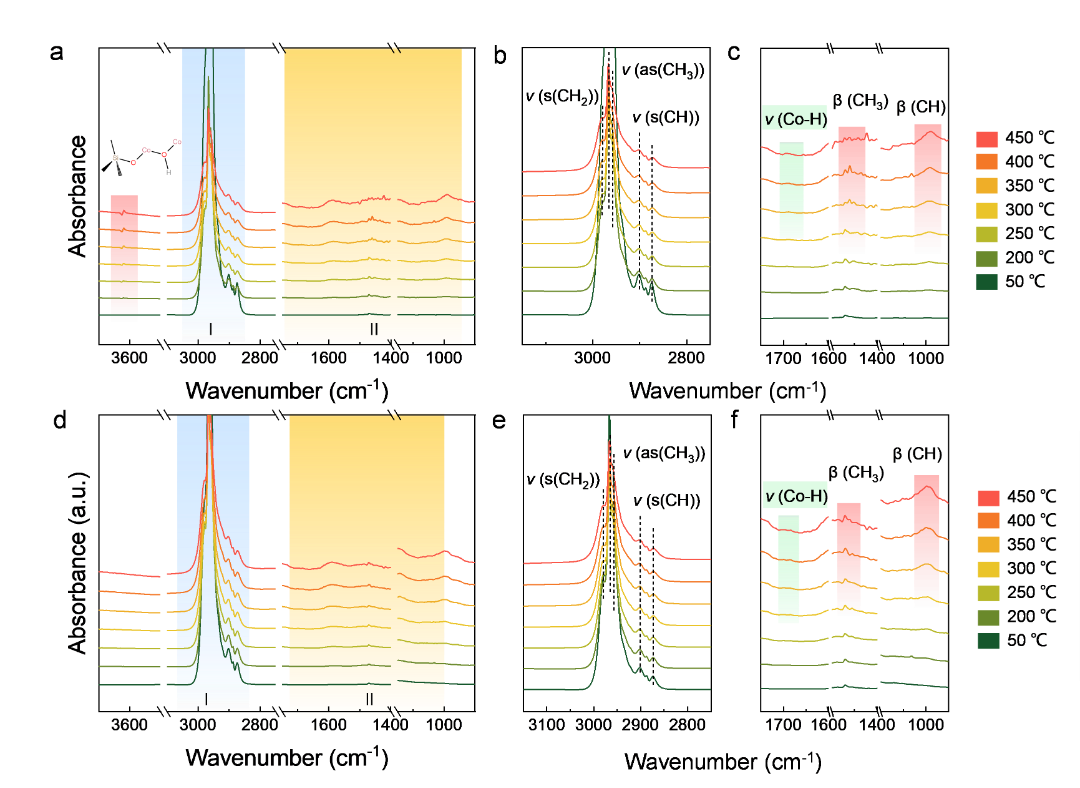
Figure 4. In-situ infrared spectra of propane for different catalysts. (a-c) CoOx@MFI。 (d-f) Co3O4/ MFI。
In-situ FT-IR showed that the vibrational peaks of the Co-H species (1690 cm-1) were observed in both the CoOx@MFI andCO3O4/MFI catalysts, and in particular in the CoOx@MFI, the CoOH* The vibrational peak of the species (3622 cm-1), which provides solid evidence for a rational explanation of the reaction mechanism of PDH. Combined with other characterization evidence and DFT calculations, it was found that there was local polarization in the CoOx@MFI catalyst, and the electrons were transferred from the Co to the O atom, thereby reducing the electron density at the Co site, promoting the formation of the active Co-O group and activating the first C-H bond. The stable Co-O group of MFI zeolite not only enhances the stability of C3H7* at the Co site, but also makes the free H* group firmly anchored at the O site. The methods of collaborative computation and experimental research in this work will provide a new perspective for the rational design of target cobalt-based catalysts, so as to achieve efficient PDH.
Cite This: https://doi.org/10.1021/acscatal.3c04113
Xi Wang, a professor and doctoral supervisor at the School of Physical Science and Engineering, Beijing Jiaotong University, has been engaged in the efficient production and utilization of green hydrogen for a long time, and has established a research route of "effective domain design strategy, catalyst design and controllable preparation, catalyst engineering and process optimization", which has been applied to residual oil extraction, propane hydrogen production, and fuel cells. He has proposed an effective domain design strategy to guide the precise design of catalysts, carried out an industrial demonstration of a 100,000-ton high-efficiency hydrogen production catalyst, developed a commercial fuel cell stack, and industrialized a new tonn-class lithium-ion battery electrode material. He has presided over the key R&D program of the Ministry of Science and Technology, the cultivation project of the major research plan of the National Science Foundation of China, and the science and technology cooperation project of Sinopec. At Nat. Nanotech、JACS、Angew. Chem.、Adv. Mater.He has published more than 160 SCI papers in journals such as Joule, and has been cited more than 14,000 timesFactor 66. He was invited to serve as an editorial board member of iScience (a sub-journal of Cell) and an associate editor of Cellulose Science and Technology. In 2021, the team's effective domain design related achievements were selected into Chapter 3 of the 2021 Science Development Report, and were introduced as "Representative Achievements of Chinese Scientific Research in 2020".
Anhui Absorption Spectrum Instrument Equipment Co., Ltd. is led by experts, based on the background of synchrotron radiation in the field of absorption/emission spectrum of more than 10 years of technical research accumulation, the development of standardized desktopX-ray absorption/emission spectroscopy equipment. Focus on X-ray absorption/emission spectroscopy technology and spectroscopic instrument development, to provide scientific researchers with professional absorption/Emission spectrum technology solutions, adhering to the technical pursuit of "let XAFS into the laboratory", research and absorption/ Emission spectrum technology, carry forward the spirit of craftsmanship and modern scientific innovation, and persistently promote the research and development of X-ray technology and equipment. As a professional XAFS person, we have been making professional XAFS equipment and are committed to helping you XAFSFreely.