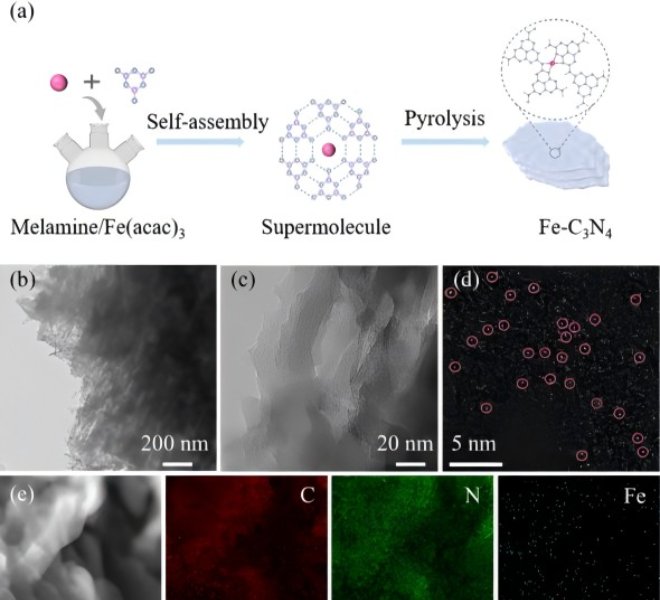
Anhui absorption spectrum RapidXAFS facilitates the characterization of 2D Co functionalized vermiculite films, and the scientific research results were published in Nature Communications
Freshwater scarcity is a major global challenge that is likely to escalate in the near future as uncontrolled population growth, climate change and water pollution intensify. In the urgent need to increase the supply of fresh water beyond the existing water cycle, advanced water treatment technologies to obtain fresh water from non-traditional water sources such as seawater and various types of wastewater have become particularly important. Compared to traditional distillation, evaporation, adsorption, and degradation methods, advanced membrane technologies are attracting attention due to their low energy consumption, high efficiency, and smaller land occupation and carbon footprint. However, the "trade-off" effect between permeability and selectivity of membranes remains a major obstacle to their commercial application.
Recently, the team of Professor Zhang Zhenghua of Tsinghua University Shenzhen International Graduate School published an article entitled "Nature Communications" in the internationally renowned academic journal Nature CommunicationsResearch paper "Overcoming the permeability-selectivity challenge in water purification using two-dimensional cobalt-functionalized vermiculite membrane".
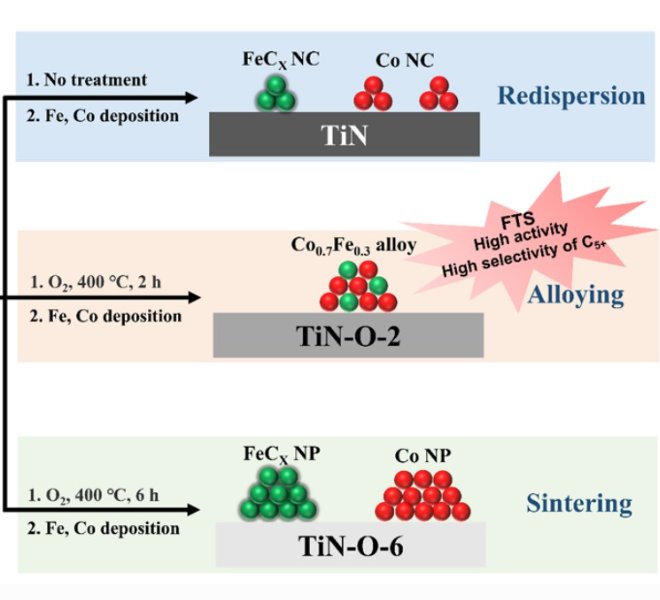
The authors propose an innovative approach that aims to break through the "trade-off" effect of membrane permeability versus selectivity. The method uses a multifunctional layered membrane (Co@VMT) assembled with Co-loaded 2D vermiculite nanosheets that enables simultaneous advanced oxidation (AOP) degrading of contaminants and membrane filtration. The catalyst within the 2D lamellar membrane not only increases the interlayer and/or intralayer spacing to increase water flux, but also ensures direct degradation and mineralization of organic contaminants. Co@VMT membrane exhibits high water permeability of 122.4 L.m-2.h-1.bar-1, which is a value for ordinary VMT membranes (1.1 L.m-2.h-1.bar-1)of two orders of magnitude. In addition, Co@VMT membranes, as carriers for the advanced oxidation process of nanofluids, can efficiently degrade a variety of organic pollutants (including dyes, drugs, and phenols) by in-situ activation of persulfate (PMS), exhibiting close to 100% degradation efficiency and stability for more than 107 hours, even in real water environments. What's more, the Co@VMT membrane combined with the PMS system can ensure that the effluent is safe and non-toxic, and the membrane concentrate enriched with contaminants will not be produced during the filtration process, which is completely different from the traditional VMT membrane based on the molecular size sieving mechanism, which has the problem of contaminant-enriched membrane concentrate when treating wastewater. This research provides a general design blueprint for the development of multifunctional nanofluid catalytic membranes, paving the way for the development of next-generation nanofluid catalytic membranes, which is expected to effectively overcome the long-standing "trade-off" effect of membrane permeability and selectivity in the water treatment process, and bring revolutionary progress to the field of water treatment.
Using XRD and AFM, the authors demonstrated the successful conversion of bulk phase into monolayer VMT nanosheets. HRTEM images show that Co is crystalline, and SEM shows that the membrane has a relatively smooth surface and a layered structure in cross-section. XPS spectra of Co2p show characteristic peaks of Co2p3/2 and Co2p1/2 as well asCo3+(795.95 eV and 780.18 eV) (37.69%) andCo2+ (782.18 eV and 797.48 eV). In order to further characterize the properties of the Co@VMT, the authors used the benchtop X-ray absorption spectrometer RapidXAFS from Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd. to analyze the local electronic and coordination structures of the membranes, and the XANES results showed that the Co K side was located at CoO and Co3O4, very different from Co foil, the Co atom is not a metallic cluster, but has a positive charge, and its oxidation state ranges between +2 and +3. The EXAFS results show two peaks at 1.41 Å and 2.37 Å, corresponding to the Co−O and Co−Co bonds, respectively. Curve fitting of Co K-edge EXAFS showed that the average coordination numbers for Co−O and Co−O−Co were 5.74 and 0.68, respectively, which was comparable to pure Co3O4 or Co(OH2), indicating the effect of the interaction of Co atoms with oxygen-containing groups attached to VMT nanosheets.
Figure 1. Schematic diagram and characterization of the synthesis process of Co@VMT nanosheets and Co@VMT films
The authors observed a typical membrane permeability-selectivity trade-off effect. VMT membranes maintain high efficiency in the removal of ranitidine (95.8%) while having a low water permeability (1.1 L.m-2.h-1.bar-1).。 In contrast, the loading of the Co nanocatalyst significantly increases the water flux of the VMT membrane. The coupling of Co@VMT membrane and PMS achieves high water flux while completing the complete degradation and mineralization of organic pollutants through a unique nanoconfined catalytic process. The Co@VMT membrane/PMS system achieved nearly 100% contaminant removal and high water permeability of 122.4 L.m-2.h-1.bar-1. Through pressure-driven continuous flow experiments, the authors demonstrated that the Co@VMT membrane/PMS system was able to operate robustly for up to 107 hours with a stable water permeability of 122.4 L.m-2.h-1.bar-1and nearly 100% ranitidine degradation efficiency. In addition, the authors investigated the effects of different pH conditions on the performance of Co@VMT membrane/PMS systems. When the pH of the solution was raised from 6 to 9, the degradation efficiency of ranitidine remained close to 100%, while the water permeability remained stable at 122.4 L.m-2.h-1.bar-1. This indicates that the Co@VMT membrane/PMS system has good environmental adaptability.
图2. Co@VMT膜/PMS系统的膜渗透性-选择性的性能评估
To further unravel the catalytic mechanism of Co@VMT membrane/PMS systems, the authors used DFT calculations and a series of specialized experiments, including electron paramagnetic resonance (EPR) and ROS quenching experiments. The adsorption energies (Eads) of PMS molecules on the (100) and (110) sides of α-Co(OH)2 and the (111) sides of Co3O4 were -3.93, -4.48 eV, and -3.32 eV, respectively, indicating that α-Co(OH)2 were exhibitedand Co3O4 can spontaneously activate PMS. After adsorption, the elongation of the O-O bond of PMS molecule suggests spontaneous dissociation of PMS and conversion to ROS for degradation of organic pollutants. The •OH and SO4•– radicals in the Co@VMT membrane/PMS system were detected by EPR experiments, and the DMPO−• produced by the membrane/PMS system were Co@VMT OH and DMPO− SO4•–The signal intensity is significantly higher than that of Co@VMT nanosheet/PMS systems. This result indicates that the interlaminar/intralaminar confined nanochannels within the membrane promote the full contact between PMS and the catalytic active site of Co, resulting in the generation of more reactive free radicals. Quenching experiments further confirmed the types of active substances and their contribution to pollutant degradation. Ethanol (EtOH) is used as a quencher for •OH and SO4•–, and tert-butanol (TBA) is used to quench •OH. The degradation of ranitidine was inhibited by 75.04% and 70.77%, respectively, in the presence of EtOH and TBA, respectively, indicating that •OH free radicals played a major role in the degradation of pollutants. Through these studies, the authors not only reveal the efficient catalytic mechanism of the Co@VMT membrane/PMS system, but also provide valuable strategies for the degradation of organic pollutants using similar nanoconfined spaces.
Figure 3. Reveal the molecular mechanisms of free radical generation and pollutant removal through DFT simulation and active substance identification
Anhui Absorption Spectrum Instrument Equipment Co., Ltd. is led by experts, based on the background of synchrotron radiation in the field of absorption/emission spectrum of more than 10 years of technical research accumulation, the development of standardized desktop X-ray absorption/emission spectrum equipment. Focusing on the development of X-ray absorption/emission spectrum technology and spectroscopy instruments, providing professional absorption/emission spectrum technology solutions for scientific researchers, adhering to the technical pursuit of "bringing XAFS into the laboratory", delving into absorption/emission spectrum technology, carrying forward the spirit of craftsmanship and modern scientific innovation, and persistently promoting the research and development of X-ray technology and equipment. As a professional XAFS person, we have been making professional XAFS equipment and are committed to helping you XAFS freedom.