The highly sensitive RapidXAFS 1M helped unravel the relationship between the electron-localized structure of the active site of the bifunctional oxygen electrocatalyst and the catalytic activity
For the growing energy demand, it is essential to develop advanced energy conversion and storage devices with high energy output and long life. Among them, rechargeable zinc-air batteries (ZAB) have attracted extensive attention in the field of energy storage due to their advantages of high energy density, environmental friendliness, and high safety. However, rechargeable ZABs are limited by slow oxygen evolution reaction (OER) kinetics and insufficient oxygen reduction reaction (ORR) activity, which makes it difficult to be applied on a large scale. Therefore, the development of efficient bifunctional oxygen electrocatalysts to accelerate slow OER/ORR kinetics is the main work in this field, and various novel preparation methods are emerging one after another.
At present, noble metals (PGMs) are widely used as electrocatalysts in the field of oxygen electrocatalysis, mainly including Pt-based ORR catalysts and Ir/Ru-based OER catalysts. However, these PGM catalysts have largely limited their potential applications due to their high cost, limited availability, and insufficient bifunctional catalysis. The non-noble metal transition metal-nitrogen-carbon (TM-N-C, where TM stands for Ni, Co, Fe, etc.) electrocatalyst has the advantages of low cost, high activity and significant stability, so TM-N-C was researched and developedHigh-performance electrocatalysts that catalyze both ORR and OER as an alternative to PGM are of great significance.
Recently, the team of Professor Xu Chengyan of Harbin Institute of Technology designed, developed and designed a synthesis of N-doped carbon nanotubes (NCNTs) combined with Co-N-C elongated hexagonal nanosheets by metastable bimetallic ZnCo-ZIF-L topological chemical phase conversion and subsequent pyrolysis method (denoted as Co-N-C@NCNTs), Co–N–C@NCNTs with excellent bifunctional ORR/OER performance, using this catalyst as a rechargeable ZAB for bifunctional cathode catalysts with a maximum power density of 237.8 mW cm-2, available at The work was published in Materials Chemistry Frontiers for 130 hours of stable cycling at 5 mA cm-2, equivalent to 850 discharge-charge cycles, demonstrating excellent charge-discharge performance and stability.
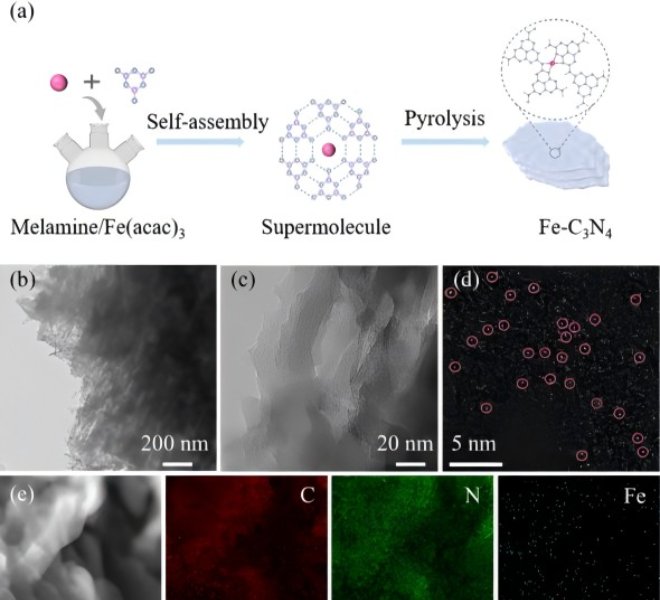
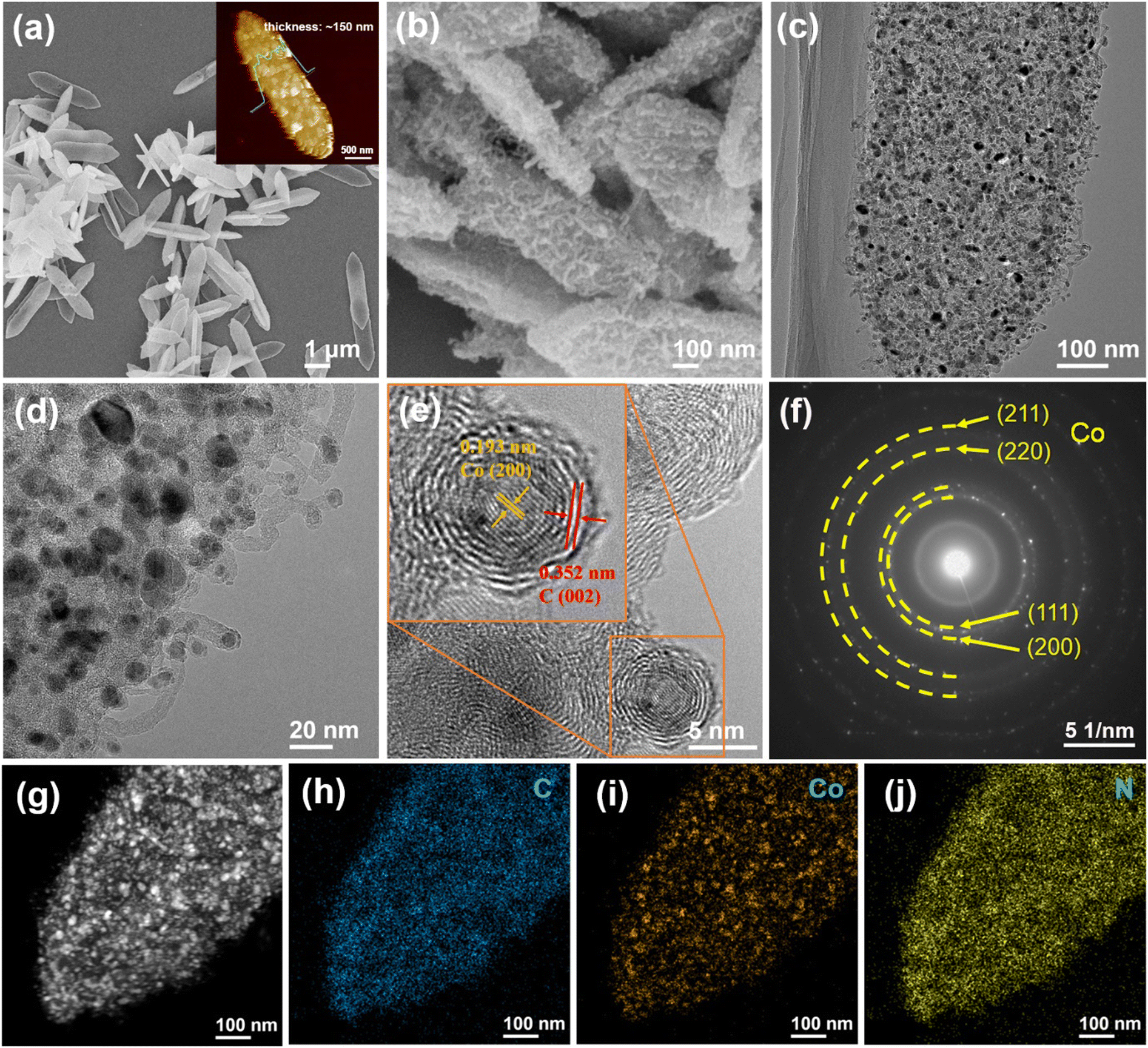
Fig.1 Microstructure characterization of Co–N–C@NCNT
Studying the microstructure of Co-N-C@NCNTs by TEM characterization revealed that a large number of carbon nanotubes grew on the carbon matrix, and the Co nanoparticles were encapsulated in the carbon matrix and the tips of each carbon nanotube (Fig. 1c), which effectively avoided the sintering of the active Co to improve the catalytic stability under harsh conditions. The specific surface area of Co-N-C@NCNTs (Fig. 2b) can indicate that it has a mesoporous structure, which helps to fully expose the catalytic site and improve the efficiency of electron transport. The XPS results confirmed that there were three main peaks in Co-N-C@NCNTs at 778.5 eV, 779.6 eV and 781.7 eV, which belonged to the metallic Co, Co-O and Co-N bonds, respectively.
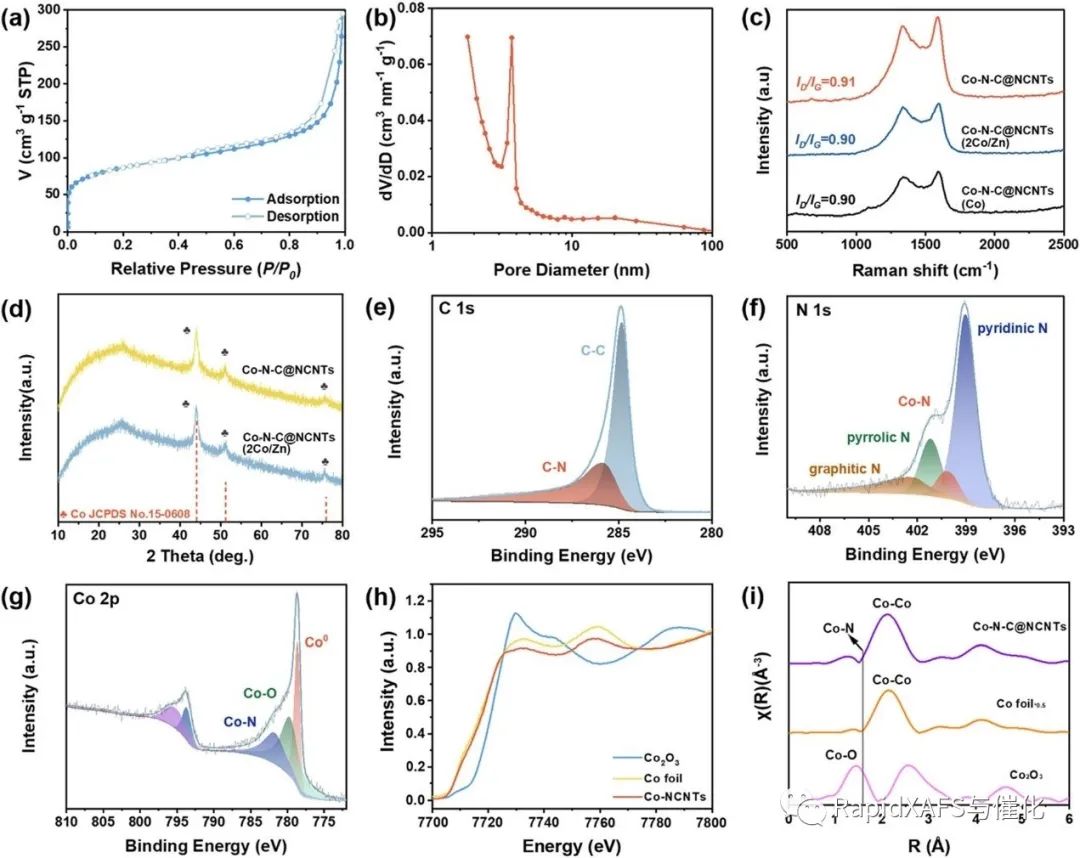
In order to further characterize the local coordination and electronic structure of the Co active center after pyrolysis, the authors performed XAFS spectroscopy characterization in the desktop X-ray absorption fine structure spectrometer RapidXAFS 1M of Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd. The Co–N–C@NCNTs X-ray absorption near-edge structure (XANES) curve of the Co–N– is slightly positively shifted compared to the zero-valent Co foil, indicating that some of the Co atoms in the Co–N–C @NCNTs are in the oxidation state (Fig. 2h). )。 The Fourier transform (FT) EXAFS spectra of Co–N–C@NCNTs, Co2O3, and Co foils are shown in Figure 2i. In addition to the main peak that is a Co-Co bond at ~2.2 Å, Co-N-C@NCNTs has a shoulder peak of Co-N bond at ~1.6 Å, indicating the generation of a Co-Nx structure in an N-doped carbon matrix. The combined XPS and XAFS results show that a small fraction of Co is isolated from each other to form an atomic Co-Nx structure in the nitrogen-carbon framework, which can be used as an active site for ORRs.
Fig.2 Structural characterization of Co–N–C@NCNT
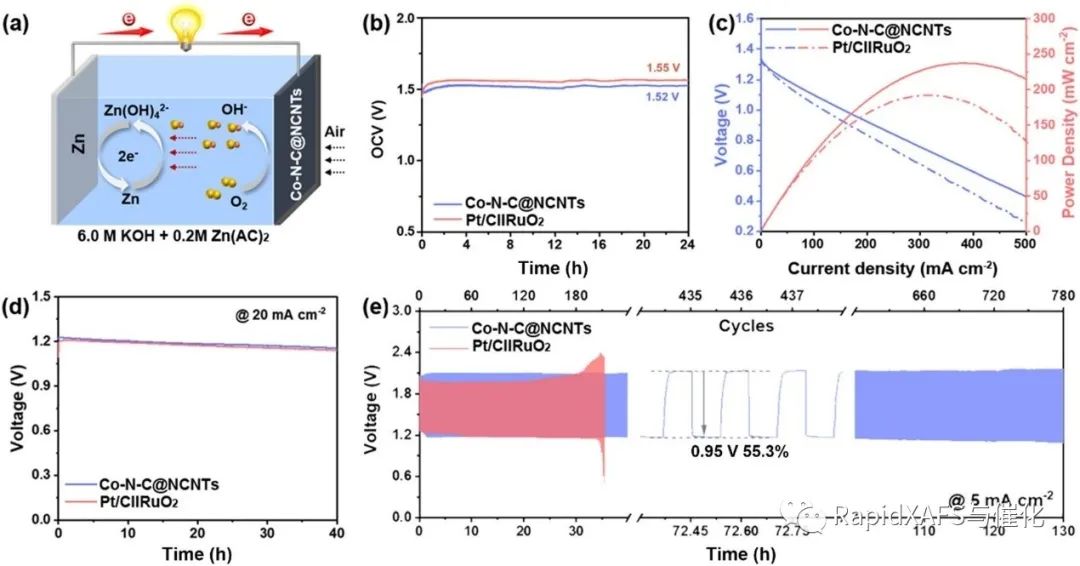
To evaluate the practical performance of the assembled rechargeable ZAB with Co-N-C@NCNTs as an air cathode catalyst, the authors composed a rechargeable ZAB loaded with Co-N-C@NCNTs as the cathode, polished zinc plate as the anode, and 6 M KOH solution as the electrolyte (Figure 3a) , the pay-off curve and the response power density (Figure 3c) show that the peak power density of Co-N-C@NCNTs-based cells is as high as 237.8 mW cm-2, cycling at 5 mA cm-270The round-trip voltage is 0.95V after hours, and it still exhibits a stable charge-discharge circuit even after 130 hours, which has better stability than Pt/C-RuO2 batteries.
Fig.3 Electrochemical performance of rechargeable ZAB
In this work, we report the synthesis of a bifunctional oxygen electrocatalyst Co–N–C@NCNTs with excellent catalytic performance by metastable bimetallic ZnCo-ZIF-L topological chemical phase conversion and subsequent pyrolysis. The catalyst has a Co nanoparticle and Co-Nx structure, as well as a unique morphology composed of interwoven carbon nanotubes, which provides more exposed active sites and promotes efficient mass and charge transport, so the material exhibits excellent bifunctional catalytic performance. This work reveals the relationship between the electron domain structure and the catalytic activity of the active site of the bifunctional oxygen electrocatalyst at the atomic scale, which provides a new way for the rational design of practical application potential and long-term stable performance, and for the construction of highly active, stable and cost-effective transition metal bifunctional catalysts.
Fei-Xiang Ma, Yu-Xuan Xiong, Hong-Shuang Fan, Zheng-Qi Liu, Yue Du, Meng-Tian Zhang, Liang Zhen and Cheng-Yan Xu*. Materials Chemistry Frontiers, 2023, https://doi.org/10.1039/D2QM01288J