How can I get high-quality XAFS data in the lab? The high-performance benchtop X-ray absorption fine structure spectrometer RaipXAFS helps you do it in one step!
Background
X-ray Absorption Fine Structure (XAFS) is a basic synchrotron radiation light source and an important means to study the local structure of materials. XAFS is very sensitive to the structural and chemical environment of the central absorbing atom, so it can give the structural information of several adjacent coordination shells around a characteristic atom at the atomic scale, such as the type of coordination atom, coordination number, atomic spacing and other structural parameters, which is widely used in catalysis, energy, nanomaterials and other popular fields.
For a long time, however, XAFS could only be tested on individual synchrotron radiation sources. Due to the limited time of the light source machine, it cannot meet the testing needs of many scientific researchers. In recent years, XAFS data has become an indispensable part of top journals and magazines, resulting in more and more research groups needing to conduct XAFS testing. With the idea of bringing XAFS technology into every lab, the Institute of High Energy Physics of the Chinese Academy of Sciences and the University of Science and Technology of China have jointly launched a new benchtop X-ray absorption fine structure spectrometer, RaipXAFS. With extremely high sensitivity and light source quality, RaipXAFS obtains XAFS data comparable to synchrotron radiation light source XAFS, and realizes valence analysis and coordination structure analysis of elements.
Figure 1. RaipXAFS benchtop X-ray absorptive fine structure spectrometer
RaipXAFS Advantage:
High performance: 1wt% content sample test completed in 1 hour;
High quality: Provide scientific research grade high-quality XAFS data;
Energy range: 4.5-15 keV, expandable to 19 keV, 4.5-25 keV (high energy).
High throughput: > 1,000,000 photons/sec@8keV, up to 2,000,000 photons/sec@8keV
Test elements: 3d, 5d, rare earth elements, Ru, Rh, Pd and other 4d transition metal elements (high energy type);
Benchtop design, XFAS test can be completed without synchrotron radiation light source, which can meet the daily sample analysis in the laboratory;
Experimental in-situ test (high temperature and high pressure, low temperature and low pressure, under various atmospheric conditions) to observe the reaction process in situ;
For low-concentration sample testing, the C-N carrier can achieve 0.1wt % sample testing
A variety of models and configurations are available to meet the needs of different scientific research work;
90% of the components are autonomous and controllable, and there is no policy risk;
Low maintenance cost: no need for special personnel to maintain, operate, manage, etc.;
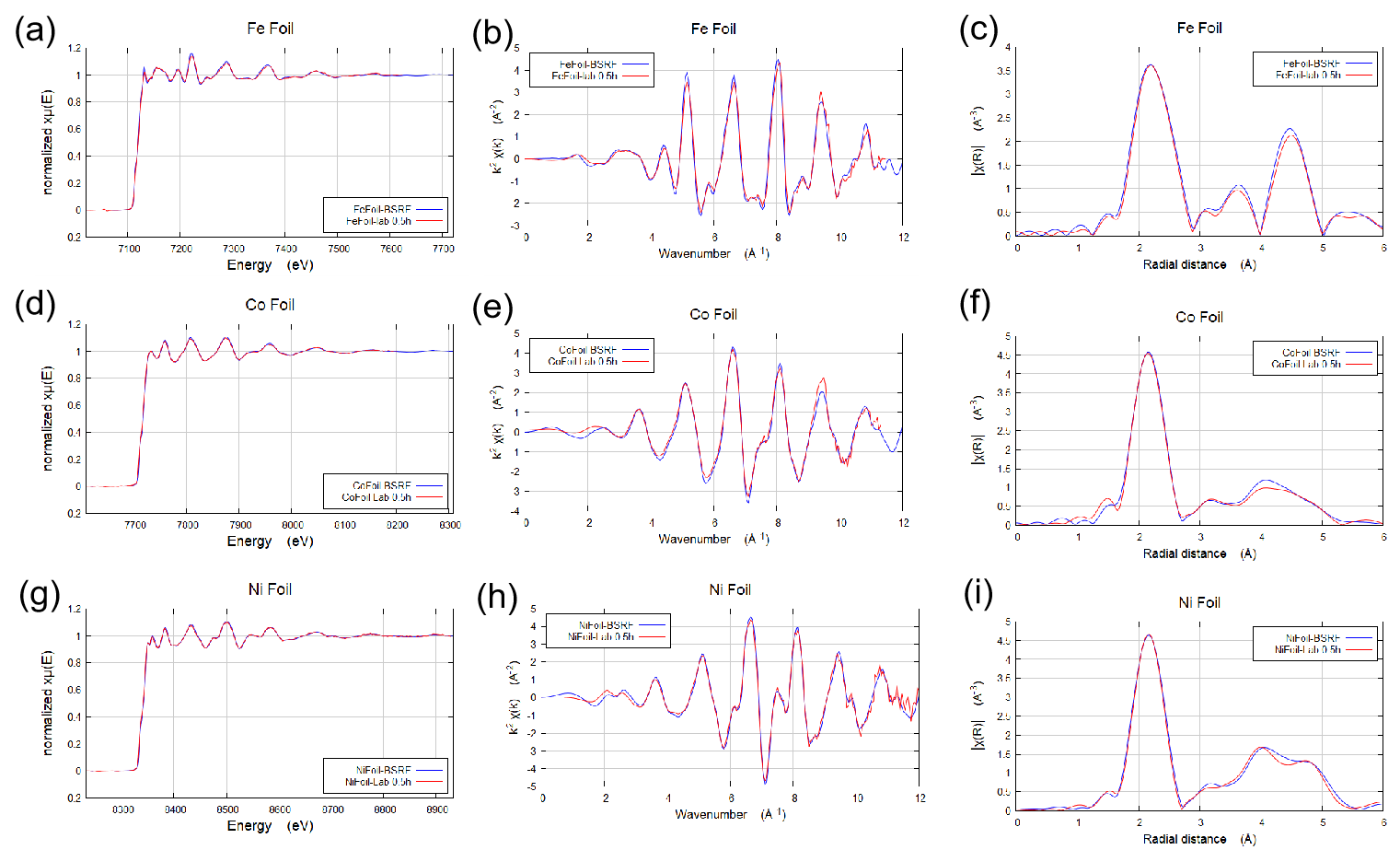
Figure 2. Comparison of Fe, Co, Ni Foil XAFS and FTRSpace data measured by RaipXAFS and synchrotron radiation sources
Applications of the RaipXAFS benchtop X-ray absorptive fine structure spectrometer
The unique benchtop design of RaipXAFS makes it possible to perform XAFS testing in the usual laboratory, avoiding the limitations of XAFS testing that could only rely on synchrotron radiation sources. Researchers can use the RaidXAFS test to analyze the valence state and coordination structure of the elements in the research materials on a daily basis in the laboratory, such as studying the valence state of the elements by X-ray absorption near-edge structure (XANES), or studying the coordination number and atomic spacing by extending the X-ray absorption fine structure (EXAFS). It is mainly used in energy storage materials, catalysts, nanomaterials, ceramics, minerals, geological analysis and other fields.
1. Energy storage materials
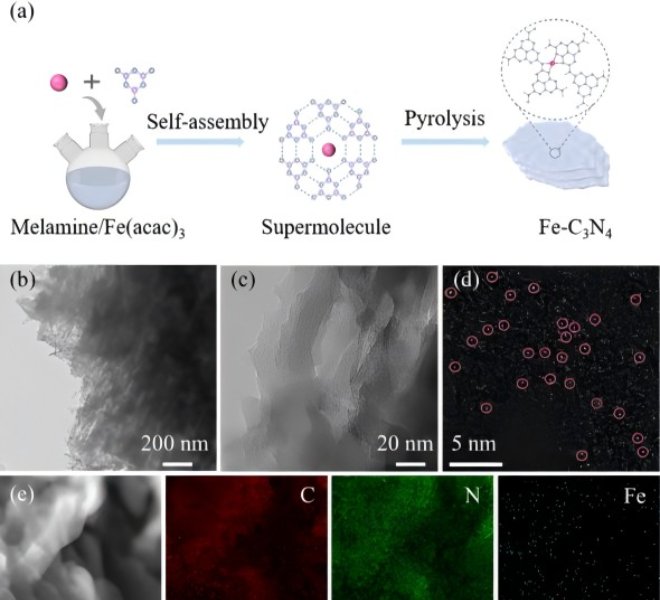
As a promising application field and research direction, energy storage materials are still in the stage of continuous exploration and improvement in terms of energy density, power and safety. The development of energy storage materials is inseparable from the characterization of XAFS technology, and the clear material structure that can be obtained through XAFS is the basis of all research. Recently, Anhui University of Technology, together with Beijing Institute of Technology and Fudan University, designed a polymer strategy to construct a nitrogen porous carbon material (Fe/Co-N-HPC) modified by N-coordinated Fe and Co binary metal atoms (SA), which showed excellent performance in capturing and catalyzing polysulfide conversion, and characterized the coordination environment of Fe and Co by benchtop XAFS spectrometer, and the results were published hereinAdv. Funct. Mater. 2022, 32, 2208666。 The data results of XANES and EXAFS can show that Fe and Co atoms are distributed separately, and the data fitting proves that Fe and Co exist in the form of Fe-N4 and Co-N4 porphyrins, respectively. Theoretical calculations show that the introduction of Co atoms can enrich the electron number of the active center of iron, thereby promoting the obvious catalytic effect of the binary metal Fe/Co SA, thereby improving the bidirectional catalysis of Li-S redox reaction in lithium-sulfur batteries.
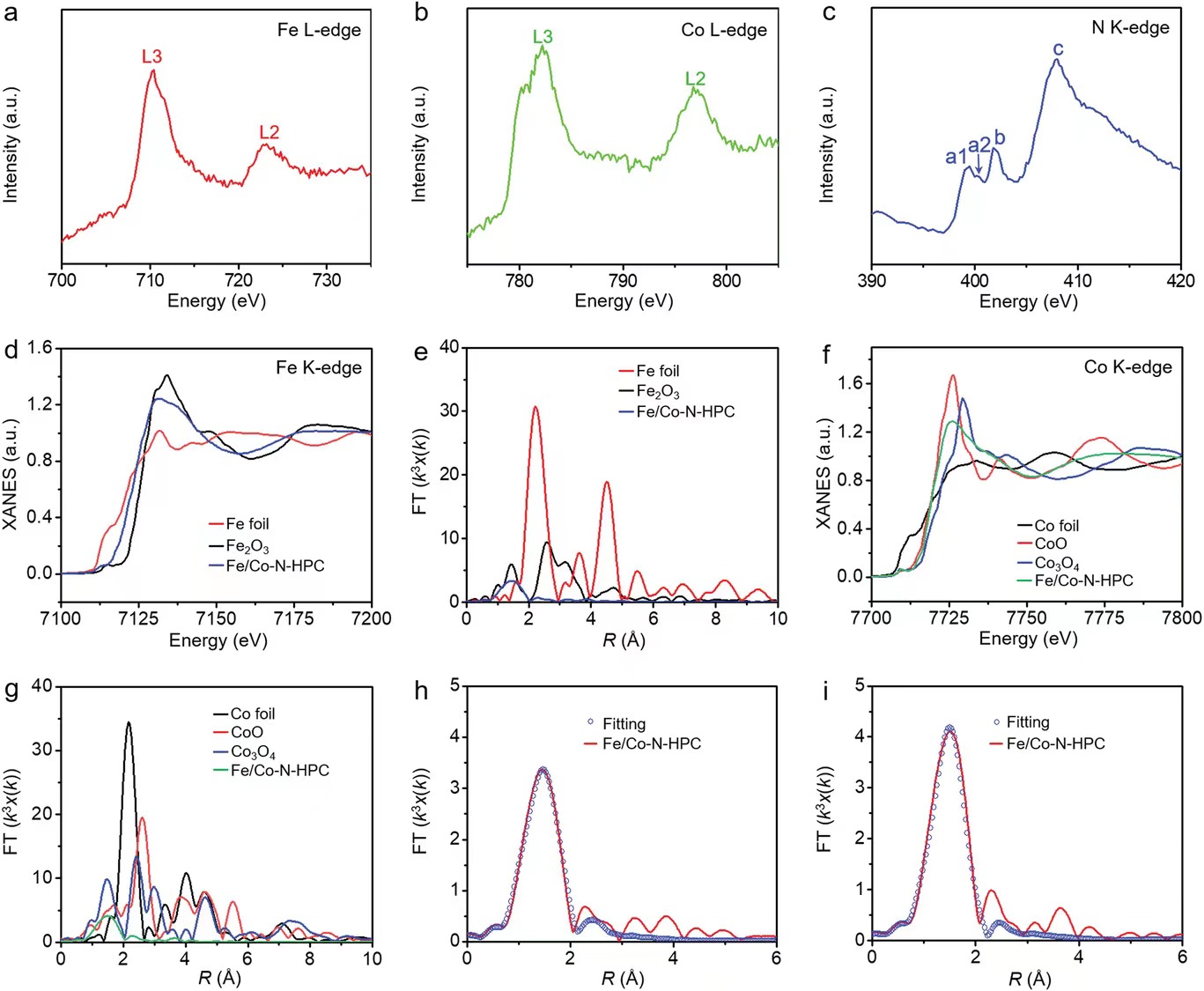
Figure 3. XANES test results for Fe/Co-N-HPC
a) Fe L-edge, b) Co L-edge, and c) N K-edge of Fe/Co-N-HPC. d) XANES spectra and e) Fourier transform (FT) of FeFoil,Fe2O3, and Fe/Co-N-HPC. f) XANES spectra and g) FT for CoFoil,CoO, Co3O4and Fe/Co-N-HPC. h) EXAFSRSpace fitting curves for Fe/Co-N-HPC for Fe and i) Co.
2. Catalysis
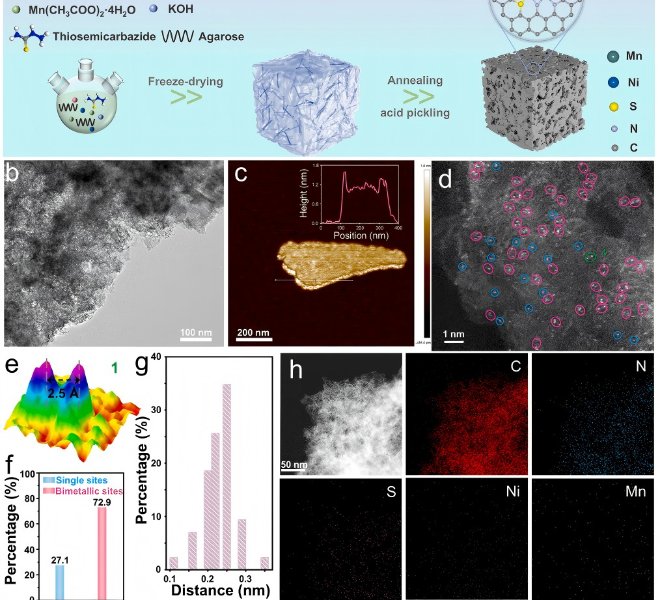
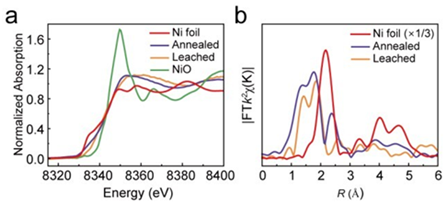
In the context of the urgent need for new clean energy, catalysts have been widely used in energy conversion and storage. Finding highly active catalysts to accelerate the process of energy conversion is the key to achieving new energy transitions. Exploring the mechanism and transformation of high-performance catalysts in the reaction process is the focus of research, and the information of transition metal element valence, coordination and electronic structure in catalysts is the basis of research, and XAFS technology is the key means to obtain such information of catalysts. Recently, Jiang Kun's research group of Shanghai Jiao Tong University, together with Professor Liang Zheng's research group, Professor Li Qiaoxia of Shanghai Electric Power University, and Dr. Xu Xu of Sinopec Petrochemical Research Institute, etc., cleverly proposed a "top-down" strategy, in which the asphalt gasification ash slag (rich in metal elements such as V, Ni, Fe, and non-metallic elements such as N, S) from Fujian United Petrochemical Co., Ltd. was synthesized in large quantities by simple annealing and pickling treatment, and passed through a benchtop XAFS spectrometerThe electronic structure of the Ni active center after annealing and pickling treatment was characterized, and the results were published in Chem. Commun., 2023,59, 611-614。 As shown in Figure 4, the k-edgeXANES spectra of Ni show that the oxidation states of Ni in the ash are between 0 and +2 valence after annealing and subsequent acid treatment, and the EXAFS spectra transformed by Fourier transform show two convex peaks at 1.4 Å and 1.8 Å indicating Ni-C or Ni-N coordination. More importantly, the Ni component in the annealed ash is not completely anchored to the carbon substrate in the form of atoms, and some of it is attached in the form of nanoclusters, which can be removed by further pickling, which is manifested in the disappearance of the Ni-Ni coordination peak in the first shell at 2.2~2.3 Å (without phase correction) in the EXAFS spectrum. The electrochemical test results in the H-type reaction cell showed that the catalyst exhibited high CO selectivity and stability in theCO2RR reaction, and could effectively drive the conversion ofCO2 to CO.
Figure 4.a) Ni K-edge XANES for annealing and leaching residual carbon compared to Ni Foil and NiO, b) FT-EXAFS spectra in R Space.
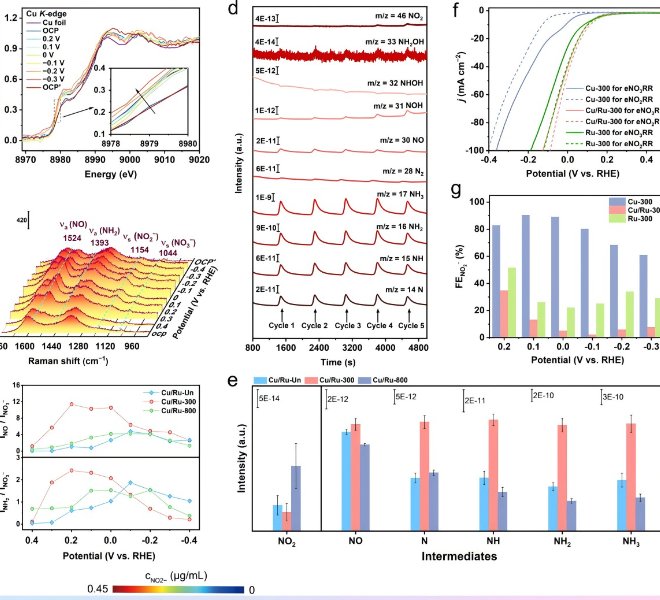
Electrochemical oxygen reduction (ORR) is an important semi-reaction in a variety of energy conversion devices, such as fuel cells and metal-air batteries. Due to the low efficiency of multi-step proton coupling in the ORR reaction process, which has become one of the bottlenecks of this kind of new energy technology, single-atom catalysts have become a hot spot in the field of catalysis due to their high activity, high selectivity, and plateau utilization rate, so the development of cheap and efficient non-noble metal bifunctional monoatomic catalyst systems is the key work in the current field. The team of Prof. Zhijun Li of Northeast Petroleum University and the team of Prof. Cheng He of Xi'an Jiaotong University designed and developed a Fe single-atom catalyst (Fe SAs/NC) with a unique coordination structure of Fe1N4O1 supported by mesoporous nitrogen-doped carbon support, and characterized the local coordination environment of Fe atoms by benchtop XAFS spectrometer, and the results were published hereAdv. Mater. 2022, 2209644。 The XANES results showed that the valence state of Fe in Fe Sas/NC was between +2 and +3, and the EXAFSR Space results showed that Fe SAs/NC produced a main peak at 1.78Å, which was related to the coordination of the first shell of Fe-N, and the absence of Fe-O and Fe-O-Fe coordination peaks in this result indicated that Fe in the catalyst was atomically dispersed. The fitting analysis showed that the Fe center was in a pentatto-coordination geometry and formed an O1-Fe 1–N4 structure with four N atoms and one O atom, and the bond lengths of Fe-N and Fe-O were 1.99Å and 2.04 Å, respectively, which confirmed the existence of Fe as an isolated atom in Fe SAs/NC.
Figure 5. Atomic structure analysis of Fe SAs/NC
a) XRD spectra. b, c) High-resolution XPS Fe2p (b) and N 1s (c) spectra. d) Fe K-edgeXANES spectra. e)FTK2-weighted Fe K-edgeEXAFS spectra. f) EXAFS fitting results in R Space (inset, schematic model ofFe1N4O1). g) 3DWT-EXAFS diagram. h, i) N (h) and C (i) K-edgeXANES spectra. j) Secondary electron tail threshold spectra
3. Environmental field
In addition to being widely used in the field of energy storage materials and catalysis, the benchtop XAFS spectrometer is also used in the environmental field. Qiu Xueqing and Qin Yanlin et al. from Guangdong University of Technology prepared zinc and nitrogen-doped nickel-based lignin-derived carbon catalysts (NiZn@NC) by solvent volatile self-assembly and in-situ reduction carbonization by solvent volatile self-assembly and in-situ reduction carbonization using pulp and papermaking waste liquid flow alkali lignin as carbon source, and characterized the local coordination environment of Ni atoms by benchtop XAFS spectrometerACS Catal. 2022, 12, 11573−11585。 Compared with the metal Ni, the Ni20Zn 1 @NC and Ni@NC catalysts exhibited stronger white peak intensity and blue shift of edge absorption, indicating that Ni produced an increased oxidation state due to coordination with N\O. With the doping of N and Zn, the Ni-Ni coordination decreased, attributed to the fact that the strength of Ni20Zn1 @NC was lower than that of Ni@NC at a radial distance of 2.50 Å radial distance of the Ni−Ni bond, suggesting that it was a result of Ni−Zn formation. Theoretical calculations show that Zn doping improves the electronic environment and structural defects of metal Ni and carbon supports, inhibits C-C cleavage to a limited extent, inhibits the formation of by-products such as methane, and achieves an ethanol conversion of 75.2% and a high alcohol yield of 41.7% on the Ni20Zn1@NC catalyst at an amination reagent/lignin mass ratio of 1:2.
Figure 6. a) Ni K-edge spectra of Ni Foil, Ni@NC, and Ni20Zn1 @NC and b) Corresponding FTK3 weighted R Space EXAFS spectra
References:
1.Adv. Funct. Mater. 2022, 32, 2208666;
2. Chem. Commun., 2023,59, 611-614;
3. Adv. Mater. 2022, 2209644;
4. ACS Catal. 2022, 12, 11573−11585;