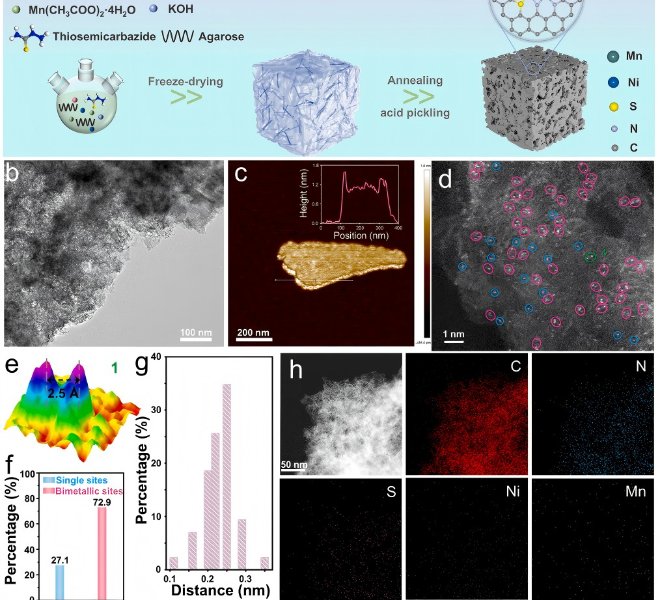
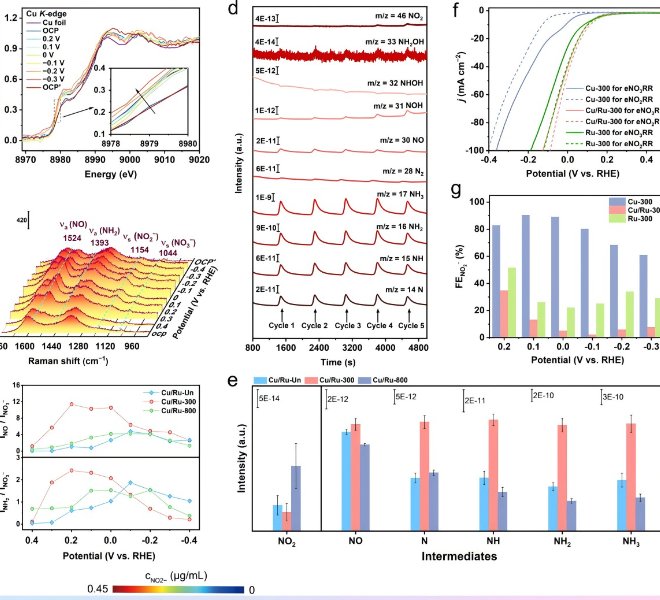
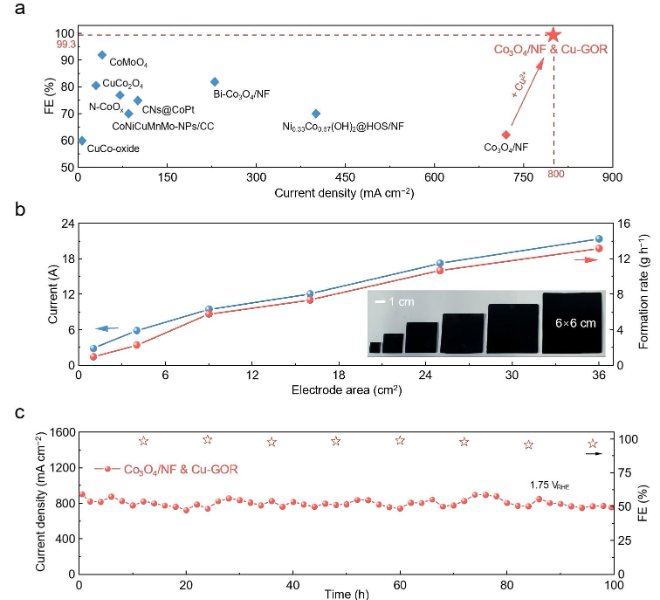
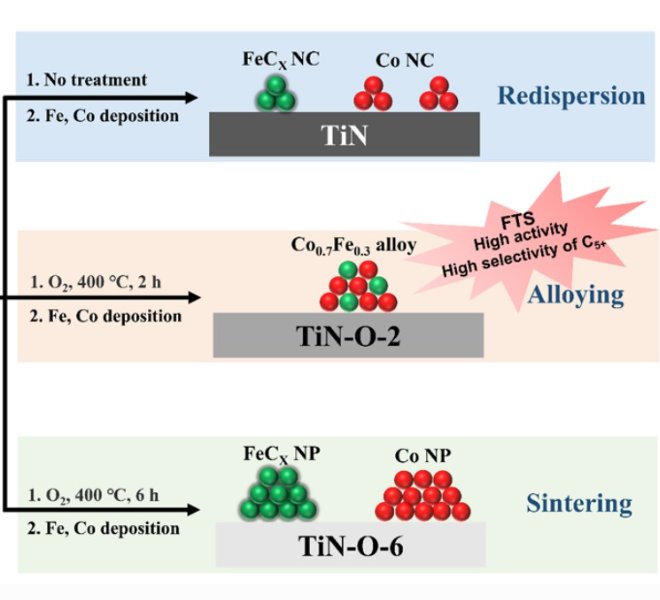
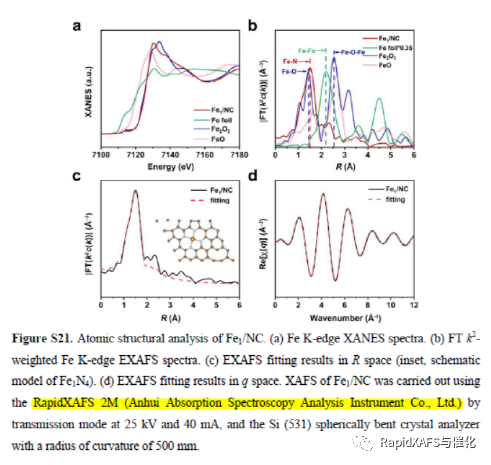
RapidXAFS 2M, an Anhui absorption spectroscopy company, assisted researchers in answering reviewer questions, and the paper was accepted by Advanced Materials
Electrochemical oxygen reduction (ORR) is an important semi-reaction in a variety of energy conversion devices, such as fuel cells and metal-air batteries. However, due to the multi-step proton coupling process involved in the ORR reaction, its efficiency is low, which has become one of the bottlenecks of this type of new energy technology. At present, a lot of work is focused on the development of ORR catalysts, and various novel preparation methods are emerging one after another. It should be noted that the ORR reaction can only be carried out effectively at the gas-solid-liquid three-phase interface. In the development of ORR catalysts, it is also necessary to pay attention to the problems of interfacial chemistry and mass transfer efficiency, so as to achieve a significant improvement in the performance of ORR catalysts and efficient utilization in energy conversion devices. Zinc-air batteries have the advantages of high theoretical energy density, green color, and safety, and have broad application prospects. ORR and oxygen evolution reaction (OER) play a decisive role in the performance of zinc-air batteries. At present, Pt/C in alkaline electrolytes has good ORR activity, IrO2 and RuO2It has good OER activity. However, the prohibitive cost greatly limits its potential applications. Single-atom catalysts have become a hot spot in the field of catalysis due to their high activity, high selectivity, and plateau utilization rate. However, it is rare to find a single-atom catalyst system with high ORR and OER activity at the same time. Therefore, it is of great significance to develop a non-noble metal bifunctional monoatomic catalyst system that can be comparable to the activity of noble metal catalysts.
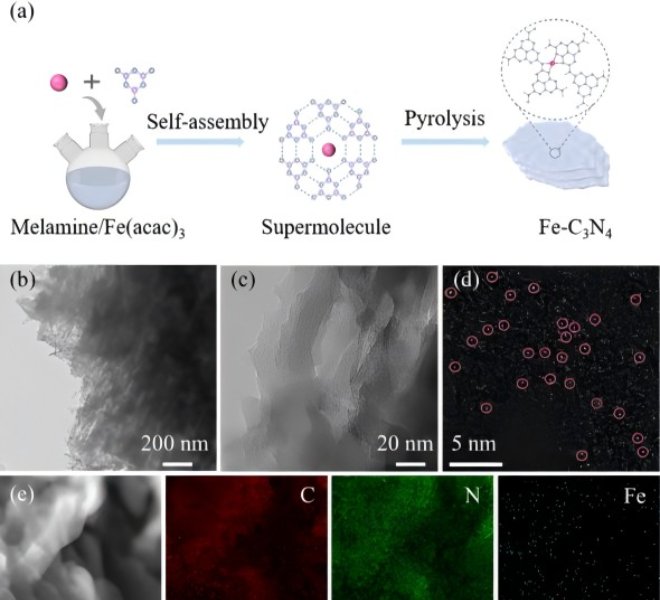
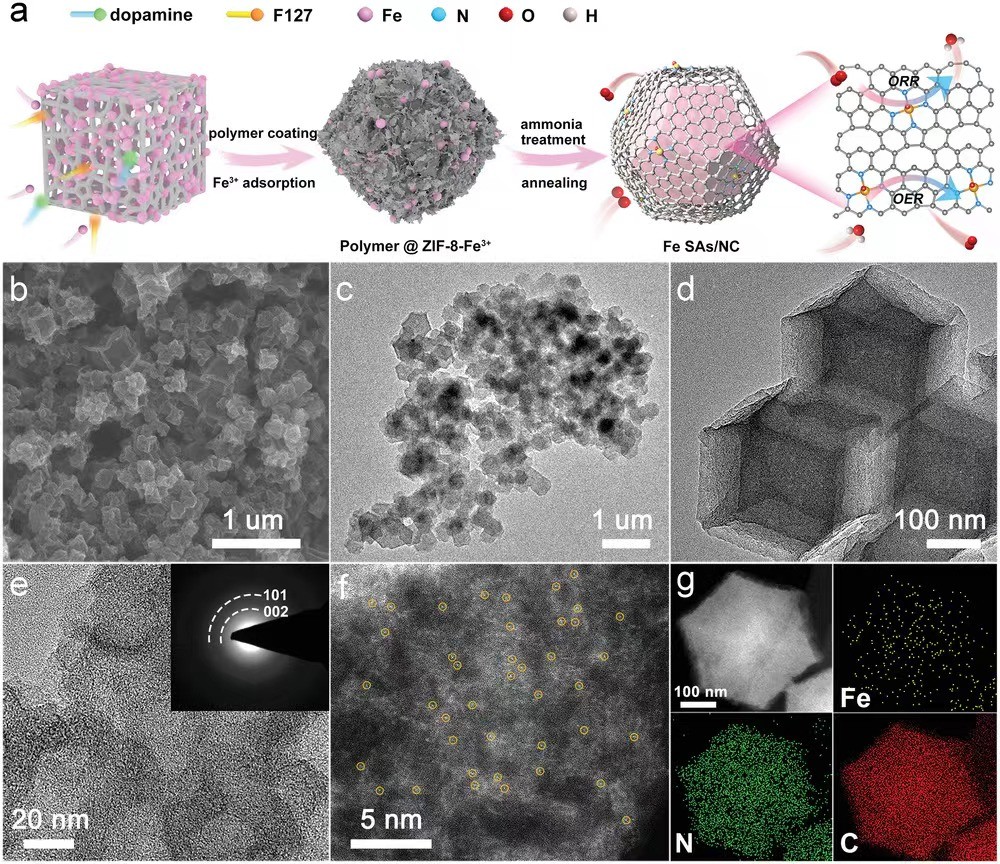
Figure 1Preparation and morphology characterization of Fe SAs/NC
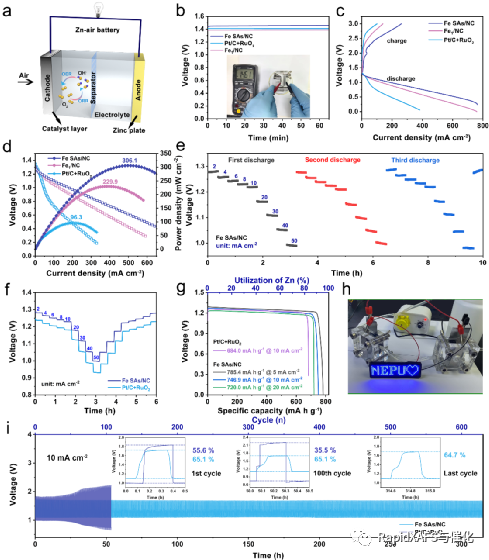
Recently, the team of Professor Li Zhijun of Northeast Petroleum University and the team of Associate Professor He Cheng of Xi'an Jiaotong University designed and developed a team with Fe1N4O1Fe single-atom catalyst (Fe SAs/NC) with unique coordination structure supported by mesoporous nitrogen-doped carbon support. The catalyst has good catalytic activity for ORR reactions in the full pH range, with half-wave potentials of 0.93 V (basic), 0.83 V (acidic), and 0.75 V (neutral), respectively. In addition, the catalyst exhibits a low overpotential of 320 mV (at 10 mA cm-2) in the alkaline OER reaction. The catalyst is assembled into a zinc-air battery, which is far superior to Pt/C+RuO 2 in terms of power density, specific capacity, and cycle stability. Theoretical calculations show that the charge distribution and electron-metal-support interaction of Fe can be effectively optimized by regulating the local coordination environment of the Fe site, which can affect the adsorption and activation of oxygen-containing intermediates at the Fe site. This work shows that the regulation of the local electronic structure of the active site is of great significance in improving the effect of electrocatalysts on the activation of small molecules. The work was published in Advanced Materials, and the first authors of the students were Dr. Siqi Ji (Northeast Petroleum University) and M.S. Sing Xu (Xi'an Jiaotong University).
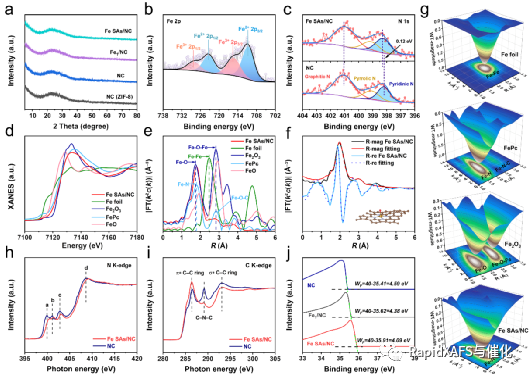
Figure 2. Atomic-level structural characterization of Fe SAs/NC
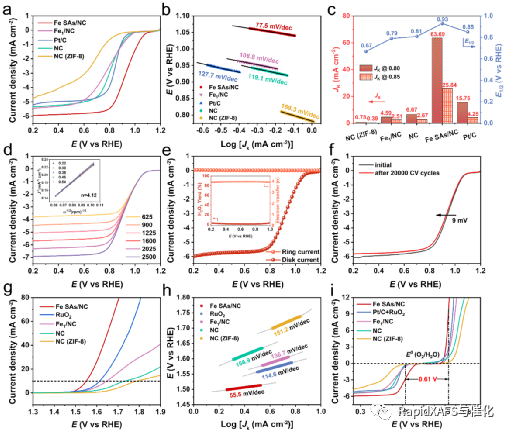
Figure 3. Basic ORR and OER properties of Fe SAs/NC
Figure 4. Zinc-air battery performance of Fe SAs/NC
Figure 5. RapidXAFS 2M supplemented withX-AFS data
(0.7% catalyst)
In this work, we report a simple and efficient synthesis method for the preparation of bifunctional oxygen electrocatalysts with excellent catalytic activity. The catalyst has a high degree of exposure to the active site, which helps to improve the utilization rate of the active site and the electron/proton transfer ability. By manipulating the local electronic environment of the active site, the charge distribution state of the active site and the electron-metal-support interaction can be effectively optimized. Promote the adsorption and activation of oxygen-containing intermediates to improve the catalytic activity of ORR and OER. This work reveals the relationship between the electron domain structure and the catalytic activity of the active site of the bifunctional oxygen electrocatalyst at the atomic scale, and provides an idea for the rational design of a low-cost, long-life and high-performance bifunctional monoatomic electrocatalyst system.
Engineering the Electronic Structure of Single Atom Iron Sites with Boosted Oxygen Bifunctional Activity for Zinc-Air Batteries;Zhijun Li,* Siqi Ji,† Chang Xu,† Leipeng Leng, Hongxue Liu, J. Hugh Horton, Lei Du, Jincheng Gao, Cheng He,* Xiaoying Qi, Qian Xu, Junfa Zhu
Advanced Materials, 2022, https://doi.org/10.1002/adma.202209644